Summary
Enterotoxigenic Bacteroides fragilis – organisms that live in the colon – secrete a metalloprotease toxin, B. fragilis toxin. This toxin binds to a specific intestinal epithelial cell receptor and stimulates cell proliferation, which is dependent, in part, on E-cadherin degradation and β-catenin–T-cell-factor nuclear signaling. γ-Secretase (or presenilin-1) is an intramembrane cleaving protease and is a positive regulator of E-cadherin cleavage and a negative regulator of β-catenin signaling. Here we examine the mechanistic details of toxin-initiated E-cadherin cleavage. B. fragilis toxin stimulated shedding of cell membrane proteins, including the 80 kDa E-cadherin ectodomain. Shedding of this domain required biologically active toxin and was not mediated by MMP-7, ADAM10 or ADAM17. Inhibition of γ-secretase blocked toxin-induced proteolysis of the 33 kDa intracellular E-cadherin domain causing cell membrane retention of a distinct β-catenin pool without diminishing toxin-induced cell proliferation. Unexpectedly, γ-secretase positively regulated basal cell proliferation dependent on the β-catenin–T-cell-factor complex. We conclude that toxin induces step-wise cleavage of E-cadherin, which is dependent on toxin metalloprotease and γ-secretase. Our results suggest that differentially regulated β-catenin pools associate with the E-cadherin–γ-secretase adherens junction complex; one pool regulated by γ-secretase is important to intestinal epithelial cell homeostasis.
Keywords: Bacteroides fragilis, Bacteroides fragilis toxin, Metalloproteases, E-cadherin, ADAM, γ-secretase
Introduction
Bacteroides fragilis are common colonic commensals that colonize the majority of humans (Moore and Holdeman, 1974). Despite comprising a minority (~0.1%) of the total fecal flora, this organism emerges as the leading anaerobic pathogen in bacteremia and is found in the vast majority, if not all, intraabdominal abscesses (Redondo et al., 1995; Polk and Kasper, 1977). Molecular genetic studies indicate that B. fragilis are highly heterogeneous and a subset of strains are termed enterotoxigenic B. fragilis (ETBF) (Franco et al., 1999). ETBFs are associated with diarrheal disease in children, adults and domestic livestock as well as active inflammatory bowel disease (IBD) (Sears, 2001; Basset et al., 2004; Prindiville et al., 2000). However, ETBFs also asymptomatically colonize up to 35% of humans (Basset et al., 2004). To date, the virulence of ETBF strains is ascribed to secretion of a 20-kDa metalloprotease toxin termed the B. fragilis toxin (BFT) (Sears, 2001).
ETBFs produce BFT as a 44 kDa preproprotein toxin that is processed by the organism and secreted as a mature 20 kDa protein (Franco et al., 2002). BFT induces marked changes in intestinal epithelial cell (IEC) function in vitro and in vivo, which are initiated when BFT binds specifically to a IEC receptor that is not E-cadherin (Wu et al., 2006). Biological activity and specific binding of BFT to IECs is mitigated by mutation of the protease motif (Franco et al., 2005; Wu et al., 2006). After IEC binding, BFT stimulates multiple host cell changes including: morphological changes in IECs as well as in polarized renal and pulmonary cell lines (Sears, 2001); reduced barrier function and increased ion transport in intestinal cell monolayers and/or human colonic epithelium (Chambers et al., 1997; Riegler et al., 1999); IEC proliferation dependent on E-cadherin expression and, in part, β-catenin–T-cell-factor (TCF) signaling (Wu et al., 2003); and cytokine secretion by IECs, most notably interleukin-8 (IL-8) (Wu et al., 2004; Kim et al., 2001; Sanfilippo et al., 2000). Consistent with these in vitro results, histopathology of animal intestinal tissue treated with ETBF or BFT reveals disruption of the epithelial layer with a mixture of acute and chronic inflammatory cells in the lamina propia (Obiso, Jr et al., 1995; Sears et al., 1995). Human intestinal histopathology from ETBF disease is not yet published.
E-cadherin is a 120 kDa glycosylated Type I transmembrane protein critical to the formation of intercellular adhesion junctions (zonula adherens in the intestinal epithelium) and to cellular signaling, proliferation and differentiation (Nelson and Nusse, 2004). The cytoplasmic domain of E-cadherin associates with β-catenin, which is in turn tethered to α-catenin and actin (Jou et al., 1995). At the cellular level, BFT induces rapid cleavage (within 1 minute) of E-cadherin yielding sequentially 33 kDa and 28 kDa cell-associated E-cadherin fragments in the human colonic carcinoma cell line HT29/C1 (Wu et al., 1998). E-cadherin cleavage by BFT releases β-catenin to the cytoplasm resulting in β-catenin nuclear localization and stimulation of β-catenin–TCF-dependent cellular proliferation (Wu et al., 2003).
Recently, basal and agonist-induced (such as by staurosporine or ionomycin) E-cadherin processing has been reported to involve sequential steps that include cleavage of the E-cadherin ectodomain by host matrix metalloproteinase 7 (MMP-7 or matrilysin) or ADAMs (a disintegrin and metalloprotease) (Marambaud et al., 2002; Ito et al., 1999; Maretzky et al., 2005; Reiss et al., 2005; McGuire et al., 2003; Noe et al., 2001; Steinhusen et al., 2001) followed by processing of the intracellular E-cadherin domain, a 38 kDa protein (in mouse embryos and A431 human epithelial cells) by γ-secretase and caspase-3 (Marambaud et al., 2002). ADAMs, a family of at least 32 transmembrane zinc-dependent host proteases, are implicated in the ectodomain shedding of various membrane-bound proteins (Blobel, 2005). Presenilin-1 or γ-secretase (termed γ-secretase hereafter) is a 48 kDa transmembrane protein that associates directly and independently with both E-cadherin and β-catenin and regulates the stability and function of the cadherins or catenin adhesion complex (Baki et al., 2001; Murayama et al., 1998; Kang et al., 2002). γ-Secretase is an aspartyl protease of the intramembrane cleaving protease family (termed iCLiPs) and appears to be a promiscuous enzyme that cleaves various Type I transmembrane domain proteins after they have undergone ectodomain shedding (Kopan and Ilagan, 2004; Wolfe and Kopan, 2004; Chyung et al., 2004). Given these new insights into cadherin biology, the goal of this study was to investigate in greater detail the mechanism by which BFT induces E-cadherin cleavage.
Results
BFT induces E-cadherin ectodomain release and HT29/C1 cell membrane protein shedding
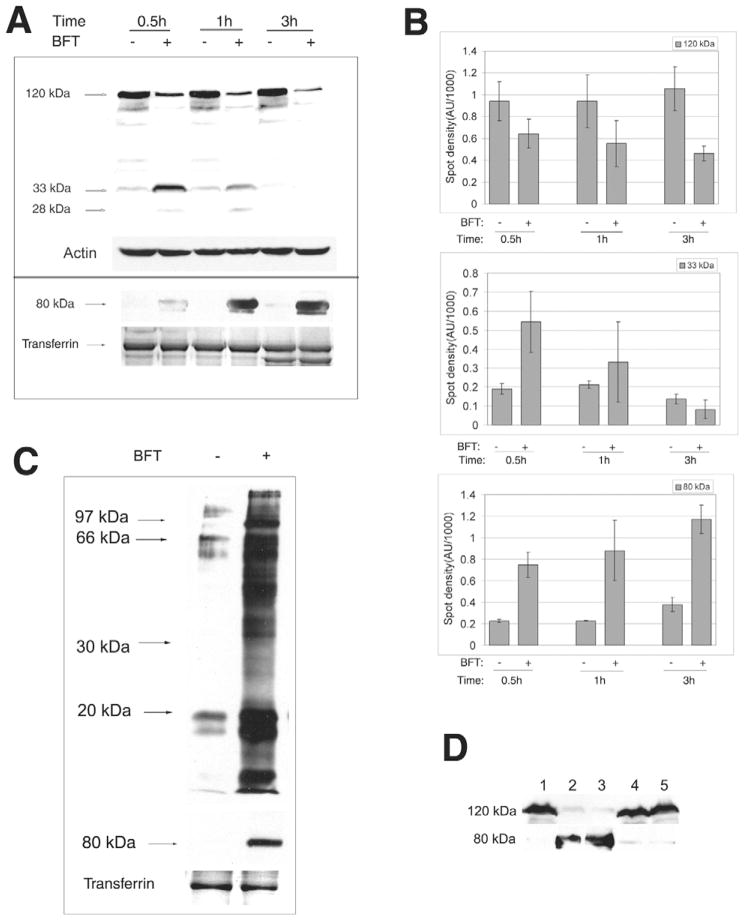
Using a polyclonal antibody to the C-terminal 25 amino acids of the cytoplasmic domain of E-cadherin (E2 antibody), Fig. 1A,B demonstrates that, consistent with previous data (Wu et al., 1998), BFT treatment of HT29/C1 cells stimulates rapid cleavage of intact 120 kDa E-cadherin yielding 33 kDa and 28 kDa cell-associated fragments of E-cadherin that are degraded over time. To determine whether the extracellular domain of E-cadherin is shed after BFT treatment, HT29/C1 cells were treated with BFT for 30 minutes to 3 hours followed by concentration of the proteins in the culture supernatant by TCA precipitation. Western blot analysis of the cell culture supernatant proteins using an antibody to the extracellular region of E-cadherin (H-108 antibody) revealed the release of an 80 kDa protein band in the cell supernatants of BFT-treated cells over time compared with a faint signal in the control samples (Fig. 1A,B). Using the H-108 antibody, the 80 kDa band was the only protein band detected in BFT-treated cell supernatants. To determine whether E-cadherin was the only cell surface protein released after BFT treatment of HT29/C1 cells, total cell membrane protein shedding was examined by biotinylation of cell membrane proteins prior to BFT treatment. As shown in Fig. 1C, unexpectedly, multiple biotinylated proteins as well as the E-cadherin ectodomain (80 kDa) were released from the HT29/C1 cell surface after 1 hour of BFT treatment when compared to untreated control cells. These results demonstrate that BFT induces shedding into the cell supernatant of a 80 kDa E-cadherin fragment as well as other, as yet unknown, membrane proteins.
Fig. 1.
BFT induces release of the E-cadherin ectodomain and shedding of IEC membrane proteins. (A) BFT induces release of the E-cadherin ectodomain. Upper panel. Cell lysates of HT29/C1 cells treated with BFT (5 nM) for 0.5 hour to 3 hours were evaluated by western blot using the E2 antibody that recognizes the C-terminal domain of E-cadherin. Actin served as an internal control for protein loading. Blot is representative of five experiments. Lower panel. TCA-precipitated cell culture supernatants of HT29/C1 cells treated with BFT (5 nM) for 0.5 hour to 3 hours were evaluated by western blot using the H108 antibody that recognizes the ectodomain of E-cadherin. Transferrin, detected by Coomasie Blue staining, served as an internal control for protein loading. Blot is representative of three experiments. (B) Normalized western blot data demonstrating significant BFT-induced cleavage of intact E-cadherin (120 kDa) on HT29/C1 cells by 0.5 hour (P<0.01) with enhanced detection of a 33 kDa cell-associated E-cadherin fragment at 0.5 hour and release of the 80 kDa E-cadherin ectodomain into cell supernatants (both P<0.001). The 33 kDa E-cadherin fragment is degraded over time (see also Fig. 1A). Data are means ± s.d. of three experiments. (C) BFT (5 nM, 1 hour) induces shedding of IEC membrane proteins as well as the E-cadherin ectodomain (80 kDa). HT29/C1 cell membrane proteins were biotin-labeled and processed as in the Materials and Methods. Transferrin stained by Coomasie Blue serves as an internal control for protein loading. Blots are representative of four experiments. (D) Cleavage of E-cadherin extracellular domain requires biologically active BFT. HT29/C1 cells were treated with purified BFT (5 nM) or culture supernatants of B. fragilis 9343(pFD340::P-bft) that expresses wild-type BFT or B. fragilis 9343(pFD340::P-bftΔH352Y) that expresses mutant biologically inactive BFT. Cell lysates and TCA-precipitated cell culture supernatants were assessed by western blot using the E2 (upper lane) and H108 (lower lane) antibodies to the E-cadherin C-terminus or ectodomain, respectively. Lane 1, untreated control; lane 2 purified BFT; lane 3, B. fragilis 9343(pFD340::P-bft); lane 4, B. fragilis 9343(pFD340::P-bftΔH352Y); lane 5, brain heart infusion broth alone.
To assess whether BFT was responsible for the release of the 80 kDa ectodomain of E-cadherin, HT29/C1 cells were treated with partially purified culture supernatants of recombinant B. fragilis strains secreting wild-type BFT or biologically inactive mutant BFT-H352Y and E-cadherin cleavage fragments were assessed in both HT29/C1 cell supernatants and lysates. Treatment of HT29/C1 cells with bacterial culture supernatants containing wild-type BFT released the E-cadherin 80 kDa ectodomain and cleavage of intact 120 kDa E-cadherin in cell lysates similar to that observed in response to purified BFT (Fig. 1D). In contrast, neither release of the 80 kDa E-cadherin ectodomain nor cleavage of E-cadherin in HT29/C1 cell lysates was detected in HT29/C1 cells treated with bacterial culture supernatants containing mutant BFT-H352Y (Fig. 1D). These results indicate that biologically active BFT is required to stimulate E-cadherin cleavage.
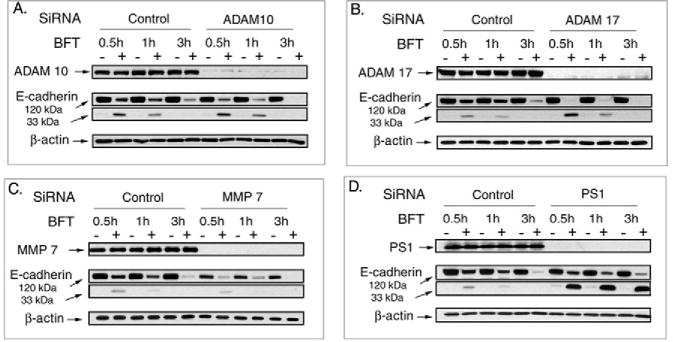
MMP-7 as well as ADAM10, but not ADAM17, mediates E-cadherin ectodomain shedding in selected epithelial cells (McGuire et al., 2003; Maretzky et al., 2005; Noe et al., 2001). In other cells, ADAM10 and ADAM17 appear to exhibit overlapping substrate profiles (Blobel, 2005). Because mutations in the protease domain of BFT ablate binding of BFT to IECs and hence the biological activity of BFT (Wu et al., 2006; Franco et al., 2005), we tested whether MMP-7 and/or ADAM10 or ADAM17 mediate E-cadherin cleavage after BFT cellular binding. We initially tested the role of MMP-7 by determining whether recombinant active MMP-7 (3.3 μg/ml) mimicked the biological activity of BFT by stimulating E-cadherin cleavage and/or cell shape changes in HT29/C1 cells over 24 hours. MMP-7-treated HT29/C1 cells developed a spindle morphology distinct from the cell rounding and separation observed in BFT-treated cells and no cleavage of E-cadherin was detected at 3 or 24 hours suggesting that MMP-7 does not mimic or mediate BFT-initiated E-cadherin cleavage. Using RNA interference to directly deplete HT29/C1 cell MMP-7 also did not inhibit BFT-initiated E-cadherin cleavage. However, HT29/C1 cells depleted of MMP-7 did not grow as well as control cells and displayed less E-cadherin (Fig. 2A). We also observed that, although expression of MMP-7 was induced by BFT after 24 hours, the active form of the protein was not detected by western blot in control or BFT-treated HT29/C1 cells, further suggesting that MMP-7 is not activated by BFT (data not shown).
Fig. 2.
MMP-7, ADAM10 or ADAM17 do not mediate BFT-initiated E-cadherin ectodomain shedding whereas γ-secretase mediates, in part, BFT-initiated E-cadherin cleavage. Cell lysates of control or siRNA-treated [MMP-7 (A), ADAM10 (B), ADAM17 (C) or γ-secretase (D) ribonucleotide pairs] HT29/C1 cells were examined by western blot for each target protein or E-cadherin (intact 120 kDa or 33 kDa degradation fragment) in the presence or absence of BFT (5 nM, 2 hours) treatment. β-actin served as an internal protein loading control. Blots are representative of three experiments.
RNA interference was also used to test whether ADAM10 or ADAM17 mediate BFT-initiated E-cadherin cleavage. Fig. 2B,C shows that active ADAM10 and ADAM17 were nearly completely depleted without modifying either basal levels of E-cadherin or BFT-initiated E-cadherin cleavage. Similar results were obtained with dual ADAM10 and ADAM17 depletion by RNA interference (data not shown).
The γ-secretase inhibitor L685,458 inhibits the proteolysis of the E-cadherin intracellular domain generated by BFT in HT29/C1 cells
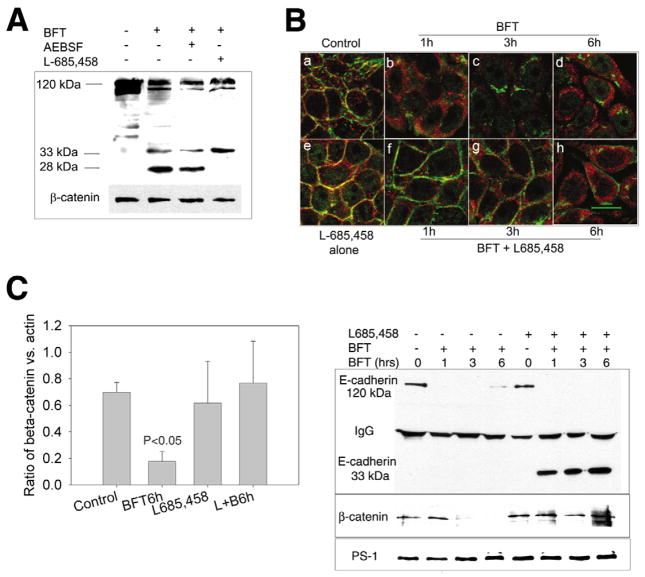
Our previous data suggested that the initial generation of the 33 kDa C-terminal E-cadherin fragment by BFT was not dependent on cellular ATP, whereas the subsequent degradation of this 33 kDa E-cadherin fragment was dependent on cellular ATP (Wu et al., 1998). This suggested that degradation of the 33 kDa E-cadherin fragment was probably attributable to cellular proteases. Recent data has identified E-cadherin as one potential substrate for γ-secretase (Marambaud et al., 2002). Thus, we tested whether degradation of the C-terminal E-cadherin fragments generated by BFT treatment of HT29/C1 cells was dependent on γ-secretase using the selective γ-secretase inhibitor, L-685,458. As shown in Fig. 3A, compared with HT29/C1 cells treated only with BFT, pretreatment of HT29/C1 cells with L-685,458, but not the β-secretase inhibitor, AEBSF, inhibits degradation of the BFT-generated 33 kDa E-cadherin fragment to a 28 kDa E-cadherin fragment. This result was verified using RNA interference to directly deplete HT29/C1 cell γ-secretase (Fig. 2D).
Fig. 3.
Inhibition of γ-secretase, but not β-secretase, blocks BFT-induced cleavage of the C-terminal domain of E-cadherin and partially β-catenin redistribution. (A) Cell lysates of HT29/C1 cells were analyzed by western blot using the E2 antibody to the C-terminus of E-cadherin. Cells were treated with BFT alone (30 minutes, 5 nM) or with inhibitors [β-secretase inhibitor, AEBSF (0.5 mM) or γ-secretase inhibitor, L-685,458 (1.5 μM)] for 30 minutes prior to BFT treatment. Molecular size markers indicate the 120 kDa intact E-cadherin and the 33 and 28 kDa degradation fragments of E-cadherin. β-catenin serves as an internal control for protein loading. Blot is representative of three experiments. (B) HT29/C1 cells were treated with BFT alone (5 nM) or in the presence of the γ-secretase inhibitor, L-685,458 (1.5 μM), followed by immunostaining with an anti-β-catenin antibody (CAT-5H10) (green) and the E2 antibody to the C-terminus of E-cadherin (red). Untreated control cells or cells pretreated for 30 minutes with L-685,458 (Panels a and e, respectively) reveal co-association (yellow) of E-cadherin and β-catenin staining. After BFT treatment, there is diffusion and dissociation of E-cadherin and β-catenin staining at 1 hour (Panel b) with progressive cytoplasmic β-catenin and E-cadherin diffusion over the subsequent 3- and 6-hour time points (Panels c,d). In cells pretreated with L-685,458, there is also dissociation of E-cadherin and β-catenin staining at 1 hour but the β-catenin signal is intense and localized at the cell membrane (Panel f). Over time, partial cytoplasmic diffusion of β-catenin occurs but with retention of a distinct β-catenin pool on the cell membrane (panels g,h). Representative of three experiments. (C) β-catenin/actin ratios in control HT29/C1 cells or cells treated with BFT (5 nM) for 6 hours in the presence or absence of L-685,458 (1.5 μM). L+B6h=L-685,458 + BFT for 6 hours. P<0.05, Control vs BFT, 6 hours. Data are means ± s.d. of five experiments (D) HT29/C1 cells were treated with BFT (5 nM) for 1, 3 or 6 hours in the presence or absence of L-685,458 (1.5 μM). Cell lysates were immunoprecipitated using an antibody to γ-secretase and the western blot analyzed using antibodies to the C-terminus of E-cadherin (E2) (upper panel), β-catenin (CAT-5H10) (middle panel) or γ-secretase (lower panel). Intact E-cadherin is 120 kDa. γ-secretase inhibition results in stable association of a 33 kDa C-terminal E-cadherin fragment and β-catenin with γ-secretase. Western blot is representative of two experiments.
Previous data suggest that γ-secretase forms a protein complex binding directly and independently (via distinct amino acid residues) to both E-cadherin and β-catenin (Baki et al., 2001; Murayama et al., 1998; Kang et al., 2002). We previously observed that BFT cleavage of E-cadherin in HT29/C1 cells results in nuclear translocation of a portion of the cellular β-catenin with proteasomal degradation of the remaining β-catenin by 3 to 6 hours after BFT treatment of the cells (Wu et al., 2003) (our unpublished data). To further examine the effect of γ-secretase inhibition on E-cadherin cleavage and β-catenin distribution in intact cells, HT29/C1 cells were treated with BFT for 1 to 6 hours in the presence or absence of L-685,458 and assessed by immunofluorescent confocal microscopy (Fig. 3B). In untreated control cells and cells pretreated with L-685,458 for 30 minutes, E-cadherin is located at the cell membrane and colocalizes with β-catenin (Fig. 3B, panels a,e). In HT29/C1 cells treated with BFT (5 nM), E-cadherin and β-catenin co-association is lost by 1 hour, with diffusion of the signals to the cytoplasm over time (panels b–d). By contrast, in HT29/C1 cells pretreated with L-685,458, BFT treatment also results in loss of E-cadherin and β-catenin co-association; however, a portion of the cellular β-catenin remains distinctly anchored on the cell membrane for at least 6 hours after BFT treatment (panels f–h). However, by 6 hours, membrane-associated β-catenin in HT29/C1 cells treated with BFT and L-685,458 is focal rather than uniform on the HT29/C1 cell membrane as at earlier time points. By contrast, a portion of the C-terminal E-cadherin fragment appears to lose its membrane staining and becomes cytoplasmic during the time course of BFT treatment even in the presence of L-685,458. Additional time course analyses indicated that total cellular β-catenin significantly declines over 6 hours in BFT-treated cells, consistent with our previously reported data (Wu et al., 2003), whereas β-catenin levels are maintained in HT29/C1 cells treated with BFT in the presence of L-685,458 (Fig. 3C,D). These data suggest that γ-secretase regulates BFT-induced cytoplasmic release of membrane-associated β-catenin as well as β-catenin degradation.
To investigate the protein-protein associations of γ-secretase, β-catenin and E-cadherin in HT29/C1 cells, γ-secretase was immunoprecipitated from HT29/C1 cell lysates treated or not with BFT (5 nM) and/or L-685,458 (1.5 μM) and co-precipitated E-cadherin, β-catenin and γ-secretase was examined by western blot. As shown in Fig. 3D, in untreated cells or cells pretreated for 30 minutes with L-685,458, both full-length E-cadherin (120 kDa) and β-catenin are associated with γ-secretase. Treatment of HT29/C1 cells with BFT over time leads to near complete loss of association of γ-secretase with E-cadherin initially and then β-catenin (Fig. 3D, left 4 lanes). By contrast, γ-secretase remains associated with the 33 kDa E-cadherin fragment and β-catenin in cells treated with BFT and L-685,458 (Fig. 3D, four right-hand lanes). Consistent with these results, Western blot analysis of Triton-X-100-soluble and -insoluble cell fractions revealed that a portion of the 33 kDa E-cadherin fragment and β-catenin are present in the Triton-X-100-insoluble cell fraction after 3 hours of treatment with BFT and L-685,458 but is not detectable in either fraction after BFT treatment alone (data not shown). Together these data suggest that γ-secretase regulates BFT-induced E-cadherin cleavage in HT29/C1 cells and that γ-secretase co-associates with a portion of the E-cadherin cytoplasmic domain and β-catenin. A portion of this complex may dissociate from the cell membrane over time (Fig. 3B, panel h).
γ-secretase regulates basal, but not BFT-induced, cellular proliferation in HT29/C1 cells
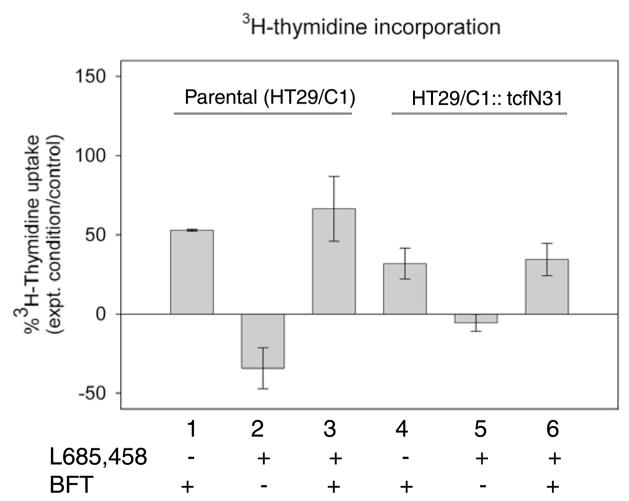
We previously reported that BFT stimulates not only E-cadherin cleavage but also nuclear localization of β-catenin and β-catenin–TCF-dependent cellular proliferation (Wu et al., 2003). To evaluate the impact of γ-secretase inhibition on BFT-induced β-catenin–TCF nuclear signaling, HT29/C1 cellular proliferation was measured using [3H]thymidine incorporation into parental HT29/C1 and HT29/C1 TCF-dominant negative (HT29/C1::TcfΔN31) cell lines. Because β-catenin nuclear signaling requires co-association with TCF, TCF dominant negative HT29/C1 cells disrupt β-catenin-dependent nuclear signaling (Wu et al., 2003). Time course analysis indicated that L-685,458 inhibited the degradation of the 33 kDa C-terminal E-cadherin fragment generated by BFT treatment of HT29/C1 cells for at least 48 hours and did not alter cell viability as assessed by trypan blue exclusion (data not shown). Fig. 4 shows that, consistent with previous data, BFT alone stimulates a significant increase in [3H]thymidine uptake versus control parental HT29/C1 cells (lane 1, P<0.001) that is not diminished when the parental HT29/C1 cells are treated with BFT in the presence of L-685,458 (lane 3 vs lane 1, P=NS). Unexpectedly, L-685,458 alone significantly reduced [3H]thymidine uptake in parental HT29/C1 cells (lane 2, −34±13%, L-685,458-treated cells vs control, P<0.001).
Fig. 4.
Effect of γ-secretase inhibition on basal and BFT-induced cell proliferation. Cell proliferation in parental HT29/C1 cells and TCF dominant negative HT29/C1 cells (HT29/C1::TcfΔN31). Cell lines were treated with BFT (5 nM) alone or in the presence of the γ-secretase inhibitor, L685,458 (1.5 μM). Results are depicted as changes in the percentage uptake of [3H]thymidine compared with control cells for each cell line. BFT induces a significant increase in HT29/C1 cell proliferation in both parental and TCF dominant negative HT29/C1 cell lines (lanes 1 or 4, P<0.001 for both cell lines vs. control) that is unaltered by L685,458 (comparisons lanes 1 vs 3 and lanes 4 vs 6, P=NS). By contrast, L685,458 inhibits parental HT29/C1 cell proliferation (lane 2, P<0.001) that is ablated in TCF dominant negative HT29/C1 cells (lane 5, P<0.01 compared with lane 2) indicating γ-secretase regulation of parental HT29/C1 cell proliferation is dependent on β-catenin/TCF nuclear signaling. Data are means ± s.d. of 3–4 experiments conducted with 3–5 replicates per experiment.
In TCF dominant negative HT29/C1 cells, as previously reported (Wu et al., 2003), BFT stimulates a significant increase in [3H]thymidine incorporation in BFT-treated cells compared with the control (Fig. 4, lane 4, P<0.001) but the [3H]thymidine uptake stimulated by BFT in HT29/C1 cells lacking functional TCF is ~40% lower than in BFT-treated parental cells (comparison between lanes 1 and 4, P<0.05). These results indicate that β-catenin–TCF nuclear signaling accounts for a portion, but not all, BFT-initiated HT29/C1 cell proliferation. Similar to the parental HT29/C1 cells, L-685,458 did not inhibit BFT stimulation of [3H]thymidine uptake in TCF dominant negative HT29/C1 cells (lane 6, BFT + L-685,458 vs lane 4, BFT alone, P=NS). By contrast, the ability of L-685,458 to inhibit [3H]thymidine incorporation in parental HT29/C1 cells is obliterated in TCF dominant negative HT29/C1 cells (comparison between lanes 2 and 5, P<0.01). These results suggest that γ-secretase does not regulate BFT-induced HT29/C1 cell proliferation but does regulate basal TCF-dependent HT29/C1 cell proliferation.
Discussion
Bacteroides fragilis are commensals colonizing the majority of humans and are the most frequent Bacteroides species identified at the mucosal surface of the colon where they probably contribute to intestinal homeostasis (Moore and Holdeman, 1974; Namavar et al., 1989; Hooper and Gordon, 2001; Coyne et al., 2005). Virulence of B. fragilis is ascribed, in part, to the unparalleled diversity of the polysaccharide capsule (Krinos et al., 2001) and, for ETBF strains, to the production of a zinc-dependent metalloprotease toxin termed BFT (Sears, 2001). We previously reported that BFT induces E-cadherin cleavage on intestinal and other polarizable epithelial cell lines resulting in adherens junction disassembly (Chambers et al., 1997; Wu et al., 1998), which initiates IEC proliferation dependent on TCF–β-catenin signaling (Wu et al., 2003).
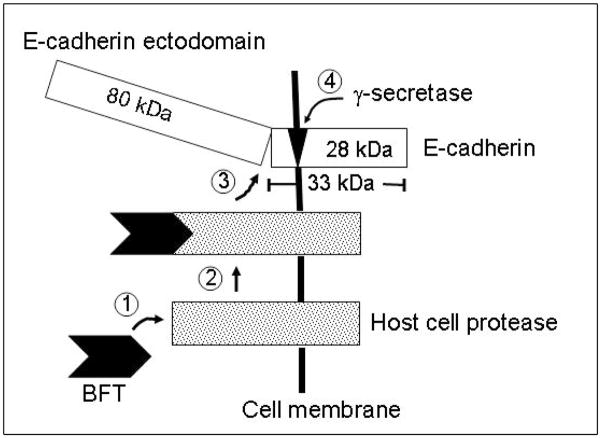
We have recently demonstrated that BFT binds to a specific HT29/C1 cell membrane receptor; that specific BFT binding requires BFT protease activity; and that the BFT receptor is unlikely to be either E-cadherin or one of the described protease-activated receptors (PAR1-4) (Wu et al., 2006). The data reported here extend our understanding of the mechanism by which BFT acts to induce disruption of the adherens junction and also suggest that signaling from the adherens junction in IECs is more complex than previously reported. Our results indicate that the proteolytic activity of BFT is necessary for shedding of the 80 kDa E-cadherin ectodomain but do not define whether BFT directly cleaves or indirectly stimulates a host cell protease to cleave the E-cadherin ectodomain. Because E-cadherin alone is insufficient to permit specific IEC binding of BFT, we propose a model of BFT action (Fig. 5) in which BFT binds to a specific host cell receptor activating a host cell protease directly or by signal transduction yielding shedding of the E-cadherin ectodomain as well as other cell membrane proteins. This model in which the host cell is proposed to mediate BFT-initiated E-cadherin cleavage is consistent with recent data indicating that E-cadherin undergoes stepwise cleavage in response to activation of cellular Ca2+-dependent signal transduction with release of the E-cadherin ectodomain (Marambaud et al., 2002; Ito et al., 1999; Steinhusen et al., 2001). Further dissection of the initial steps in BFT interaction with the cell and the role(s) of the BFT protease domain and/or cellular proteases in the mechanism of action of BFT has been limited because we have not identified a protease inhibitor that specifically inhibits eukaryotic proteases, but not BFT. Similarly, mutational analyses of BFT suggest it is a highly conserved protein without, as yet, a defined binding domain distinct from its protease domain (Franco et al., 2005; Sears et al., 2006).
Fig. 5.
Proposed model of BFT mechanism of action. BFT binds to and probably cleaves a specific host cell receptor (Wu et al., 2006), potentially activating a host cell protease that either alone or complexed with BFT then cleaves the E-cadherin ectodomain and stimulates activation of γ-secretase that cleaves the cell-associated E-cadherin remnant. Alternatively, BFT receptor binding may stimulate signal transduction activating a host cell protease that stimulates cleavage of E-cadherin.
The host cell proteases involved in ectodomain cleavage of E-cadherin have been examined in select cell lines with a role identified for MMP-7 in lung epithelium (McGuire et al., 2003) and ADAM10, but not ADAM17, in fibroblast and keratinocyte cell lines (Maretzky et al., 2005). A similar role for ADAM10 in shedding of the N-cadherin ectodomain has been reported (Reiss et al., 2005). Additional data, however, suggest that ADAM10 and ADAM17 (also known as tumor necrosis factor-αconverting enzyme or TACE) may, in some cells, modify similar substrates (Blobel, 2005). Thus, we tested, but did not identify any evidence that MMP-7, ADAM10 or ADAM17 mediates BFT-initiated E-cadherin cleavage. In particular, siRNA depletion of MMP-7, ADAM10 or ADAM17 was nearly complete but did not modify the time course of BFT-initiated E-cadherin cleavage (Fig. 2 and data not shown). In addition, ADAM10 has been proposed to regulate physiological E-cadherin cleavage in selected cells (Maretzky et al., 2005). Thus, we expected, but did not observe, increased basal intact E-cadherin levels in ADAM10-depleted cells. Our results suggest that, in contrast to other cell lines, neither ADAM10 nor ADAM17 are critical to basal or BFT-stimulated E-cadherin cleavage in an IEC model cell line, HT29/C1 cells. Additional studies using, for example, stable inducible knockouts of these enzymes in intestinal epithelium will be necessary to definitively evaluate the role of ADAMs in IEC E-cadherin processing.
Interestingly, our data show that BFT induces shedding of multiple cell membrane proteins. Although the release of cellular ligands and receptors by proteases may serve to amplify cell signaling induced by an agonist (Koon et al., 2004), a specific role of the E-cadherin ectodomain as a cell signaling agonist has not yet been described (Kopan and Ilagan, 2004). Nonetheless, it seems likely that one or more of the proteins released by BFT may act to modify the cellular response. We are presently defining the identity of the BFT-released cell membrane proteins.
After BFT stimulates shedding of the E-cadherin ectodomain, processing of the residual E-cadherin cytoplasmic domain appears to be mediated by γ-secretase, similarly to previously reported data in mouse embryos and A431 cells (Marambaud et al., 2002). Prior reports also indicate that cleavage of E-cadherin with dissociation of the adherens junction by BFT, Ca2+ signaling or staurosporine results in cytoplasmic redistribution of the nuclear signaling protein, β-catenin (Wu et al., 2003; Marambaud et al., 2002; Ito et al., 1999). γ-secretase has been proposed to inhibit cytoplasmic, but not membrane-associated, β-catenin signaling and this regulatory role has been reported in embryonic cells as distinct from the proteolytic activity of γ-secretase (Murayama et al., 1998; Kang et al., 2002). Consistent with this, loss of γ-secretase was reported to enhance β-catenin signaling and skin tumor development in vivo (Xia et al., 2001). In contrast to these results, our data suggest that γ-secretase activity positively regulates a membrane- and cytoskeleton-associated pool of β-catenin that acts in concert with TCF to contribute to basal HT29/C1 cell replication. Consistent with our results, in vivo treatment of rodents with γ-secretase inhibitors nearly halted intestinal epithelium proliferation, modified IEC differentiation and altered small intestine morphology (Searfoss et al., 2003; Wong et al., 2004; van Es and Clevers, 2005). The impact of in vivo γ-secretase inhibition on colonic morphology was not reported. However, γ-secretase inhibition did not alter BFT-induced cellular proliferation in parental or TCF dominant negative HT29/C1 cells. Together these results reveal, to our knowledge, the first evidence for two differentially regulated or heterogeneous pools of β-catenin associated with the adherens junction of IECs. BFT-induced cellular proliferation in HT29/C1 cells, although dependent on E-cadherin expression and partially dependent on β-catenin–TCF signaling (Wu et al., 2003), is independent of γ-secretase-mediated E-cadherin cleavage whereas basal cell proliferation is dependent on γ-secretase-regulated β-catenin–TCF signaling (Fig. 4).
Previous data indicate that β-catenin associates with E-cadherin and that both proteins bind to γ-secretase at distinct sites (Murayama et al., 1998; Baki et al., 2001; Kang et al., 2002). Thus, one possibility to explain our results is that BFT treatment of cells leads to a biochemical modification of a portion of E-cadherin-associated β-catenin contributing to its cytoplasmic and nuclear redistribution whereas unmodified β-catenin remains regulated by γ-secretase. Initial studies using antibodies to mono- or triphosphorylated β-catenin have not identified differences in these β-catenin species between HT29/C1 cells treated with BFT in the presence or absence of L-685,458 (S.W. and C.L.S., unpublished data). However, additional work is necessary to explore this possibility. Unlike other γ-secretase-regulated pathways, such as proteolysis of Notch, where the released cytoplasmic domain of the target protein acts as a transcriptional regulator, the E-cadherin cytoplasmic domain has not yet been identified to act as a transcriptional regulator (Kopan and Ilagan, 2004; Wolfe and Kopan, 2004). Our preliminary data suggest BFT does not upregulate expression of Notch-regulated genes (S.W. and C.L.S., unpublished data). Although mutations in γ-secretase account for the majority of early-onset familial Alzheimer’s disease (Brunkan and Goate, 2005), it is unknown whether mutations in γ-secretase contribute to IEC signaling disturbances resulting in intestinal pathology or disease.
Bacterial toxins have long served as tools to investigate normal and pathological cellular function. BFT shares sequence homology with the eukaryotic MMPs of the metzincin family and has been proposed to be the ancestral origin of the eukaryotic MMPs (Massova et al., 1998). Further studies to detail the mechanism of action of BFT will contribute to our understanding of IEC signaling pathways and cell function.
Materials and Methods
Cell lines
HT29/C1 cells are cloned cells derived from a human colon carcinoma (obtained from Dr Daniel Louvard, Institut Pasteur, Paris, France) (Huet et al., 1987; Montrose-Rafizadeh et al., 1991). The HT29/C1::TCF-4ΔN31 cell line is a derived polyclonal stable TCF dominant negative HT29/C1 cell line (Wu et al., 2003). Both cell lines were grown subconfluently in Dulbecco’s minimum essential medium (DMEM) containing streptomycin (0.1 mg/ml), penicillin (100 U/ml), G418 (0.4 mg/ml for HT29/C1::TcfΔN31 cell line only), human transferrin (10 μg/ml, Sigma, St Louis, MO) and 10% fetal bovine serum (HyClone, Logan, Utah). All culture media and reagents were purchased from GIBCO BRL Life Technologies (Rockville, MD) unless otherwise stated.
BFT purification and bacterial strains
BFT was purified from culture supernatants of B. fragilis recombinant strain I1345(pFD340::P-bft) as previously described (Wu et al., 2002). In some experiments, filter-sterilized, concentrated culture supernatants of B. fragilis recombinant strains 9343(pFD340::P-bft, secretes wild-type BFT) and 9343(pFD340::P-bftΔH352Y, secretes mutant biologically inactive BFT owing to a single nucleotide point mutation in the BFT metalloprotease domain) (Franco et al., 2005) were used for the treatment of HT29/C1 cells. Similar quantities of wild-type and mutated inactive BFT were present in culture supernatants as assessed by western blot analysis using anti-BFT antibody (data not shown).
Inhibitors and other treatments
HT29/C1 cells were washed once with Hank’s balanced salt solution before treatment with purified BFT at specified concentrations in DMEM lacking serum. Cells were incubated with the γ-secretase inhibitor (L-685,458; Calbiochem, San Diego, CA) or β-secretase inhibitor [(4-(2-aminoethyl) benzenesulfonylfluoride (AEBSF), Sigma] for 30 minutes before the addition of BFT and then continuously during the experiment unless otherwise described. Active human recombinant matrilysin (MMP-7; specific activity ≥3000 U/mg) was obtained from Calbiochem.
Antibodies
E2: polyclonal anti-C-terminal of E-cadherin antibody (provided by James Nelson, Stanford University, Palo Alto, CA); C36: monoclonal anti-C-terminal E-cadherin antibody (BD Biosciences, Palo Alto, CA); H108: polyclonal anti-extracellular E-cadherin domain antibody (Santa Cruz Biotechnology, Santa Cruz, CA); CAT-5H10: monoclonal anti-β-catenin antibody (Zymed Laboratories Inc., San Francisco, CA); polyclonal anti-α-presenilin-1-loop antibody (Calbiochem); monoclonal anti-presenilin-1-loop antibody (Chemicon International, Temecula, CA); AC-40 or AC-15: monoclonal anti-actin antibody (Sigma); monoclonal anti-biotin antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa); polyclonal anti-cytoplasmic ADAM10 or ADAM17 domain antibody (Axxora, San Diego, CA); polyclonal anti-active/latent MMP-7 antibody (Chemicon).
Immunoblot analysis and evaluation of HT29/C1 cell supernatant proteins
For western blot analyses, proteins were probed using the primary antibodies as indicated then horseradish peroxidase-coupled secondary antibodies (Jackson ImmunoResearch Laboratories) after 10% SDS-PAGE separation and nitrocellulose membrane transfer (Sambrook et al., 1989). Immunoreactive proteins were detected using Super Signal West Pico Chemiluminiscent Substrate (Pierce, Rockford, IL) and quantified by densitometry using AlphaEase™ (version 5.1; Alpha Innotech Corporation, San Leandro, CA).
To detect released E-cadherin fragments in HT29/C1 cell culture supernatants, proteins in the culture supernatants were precipitated with 10% trichloroacetic acid followed by western blot using the H108 antibody. To detect cell membrane proteins shed from cells treated with BFT, HT29/C1 cell surface proteins were biotinylated using EZ-Link Sulfo-NHS-Biotin Reagents (Pierce, Rockford, IL) as per the manufacturer’s instructions. Proteins shed into the culture supernatants were retrieved using streptavidin beads, eluted with 0.05 M glycine (pH 2.5) and detected by western blot using an anti-biotin antibody.
Cell proteins were extracted with 1% Triton X-100 in phosphate-buffered saline for 10 minutes at 4°C. The supernatant (Triton X-100 soluble fraction) was collected and the residual portion (Triton X-100 insoluble fraction) was solubilized with 1% SDS-lysis buffer (Nathke et al., 1994). The proteins from both fractions were analyzed by western blot as described.
RNA interference
Small interfering RNA (siRNA) duplex oligoribonucleotides against human ADAM10, ADAM17, MMP-7 or γ-secretase were selected and synthesized by Invitrogen (Stealth™ RNAi, Carlsbad, CA). The sequences were as follows: (i) ADAM10 sense 5′-GAGGAAAUACCAGAUGACUGGUGUA-3′, antisense 5′-UACACCAGUCAUCUGGUAUUUCCUC-3′, (ii) ADAM 17 sense 5′-GGAAGCUGACCUGGUUACAACUCAU-3′, antisense 5′-AUGAGUUGUAACCAGGUCAGCUUCC-3′; (iii) MMP-7 sense 5′-CCCGCGUCAUAGAAAUAAUGCAGAA-3′, antisense 5′-UUCUGCAUUAUUUCUAUGACGCGGG-3′; (iv) γ-secretase sense 5′-GCUCAGGAGAGAAAUGAAACGCUUU-3′, antisense 5′-AAAGCGUUUCAUUUCUCUCCUGAGC-3′. Control siRNA (Cat.No. 12935-300) was purchased from Invitrogen. Two hours after cell plating (5×104 cells/well using 24-well plate), the siRNA oligoribonucleotides were transfected into HT29/C1 cells using DharmaFECT™ 4 reagent (Dharmacon, Lafayette, CO) according to the manufacturer’s protocol followed by BFT treatment 3 days later. After washing with cold PBS, cells were lysed with RIPA buffer [Sigma; 50 mM Tris-HCl (pH 7.2–8.0), 150 mM sodium chloride, 1.0% NP-40, 0.5–1% sodium deoxycholate, 0.1% SDS] supplemented with protease inhibitors (Roche Diagnostics, Indianapolis, IN) followed by western blot analysis.
Immunoprecipitation
HT29/C1 cells were lysed in RIPA buffer and incubated with the desired antibody (1–5 μg) overnight at 4°C. Protein A-Sepharose beads (CL-4B, Amersham Biosciences, Piscataway, NJ) were added and incubated at 25°C for 1 hour. Proteins were eluted from the beads in 50 μl of 0.1 M citric acid (pH 2.5).
Immunofluorescent staining
HT29/C1 cells grown on eight-well chamber slides were fixed with 4% paraformaldehyde (Sigma) and permeabilized with 1% Triton X-100. Specific proteins were immunostained with antibodies as indicated followed by incubation with Alexa Fluor 488-labeled or Alexa Fluor 568-labeled secondary antibody (Molecular Probes, Eugene, OR). The signal was observed by single or dual channel confocal microscopy (Zeiss, LSM410). The images were processed using Metamorph (Universal Imaging, West Chester, Pa) or Photoshop 8.0.
[3H]thymidine incorporation experiments
HT29/C1 or HT29/C1::TCF-4ΔN31 cells in a 96-well plate treated with or without BFT in the presence or absence of L-685,458 were exposed to [3H]thymidine (1 μCi/ml) for 3 hours prior to the designated end of the experiment 48 hours after initiation of BFT treatment as previously described (Wu et al., 2003).
Statistical analysis
Data are expressed as means ± s.d. GraphPad Instat Version 3.05 was used for Student’s t-test or ANOVA determinations to examine for significant statistical differences considered to be P values less than 0.05.
Acknowledgments
The authors thank Dwight Derr for assistance with tissue culture and the laboratory of Anirban Maitra for assessment of Notch-regulated gene expression. Supported by grant RO1 DK 45496 (to C.L.S.) and R24 DK 64388 (PI: Mark Donowitz).
References
- Baki L, Marambaud P, Efthimiopoulos S, Georgakopoulos A, Wen P, Cui W, Shioi J, Koo E, Ozawa M, Friedrich VL, Jr, et al. Presenilin-1 binds cytoplasmic epithelial cadherin, inhibits cadherin/p120 association, and regulates stability and function of the cadherin/catenin adhesion complex. Proc Natl Acad Sci USA. 2001;98:2381–2386. doi: 10.1073/pnas.041603398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–1432. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–792. doi: 10.1111/j.1471-4159.2005.03099.x. [DOI] [PubMed] [Google Scholar]
- Chambers FG, Koshy SS, Saidi RF, Clark DP, Moore RD, Sears CL. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84 Cells) in vitro. Infect Immun. 1997;65:3561–3570. doi: 10.1128/iai.65.9.3561-3570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyung JH, Raper DM, Selkoe DJ. gamma-secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J Biol Chem. 2005;280:4383–4392. doi: 10.1074/jbc.M409272200. [DOI] [PubMed] [Google Scholar]
- Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307:1778–1781. doi: 10.1126/science.1106469. [DOI] [PubMed] [Google Scholar]
- Franco AA, Cheng RK, Chung GT, Wu S, Oh HB, Sears CL. Molecular evolution of the pathogenicity island of enterotoxigenic Bacteroides fragilis strains. J Bacteriol. 1999;181:6623–6633. doi: 10.1128/jb.181.21.6623-6633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AA, Cheng RK, Goodman A, Sears CL. Modulation of bft expression by the Bacteroides fragilis pathogenicity island and its flanking region. Mol Microbiol. 2002;45:1067–1077. doi: 10.1046/j.1365-2958.2002.03077.x. [DOI] [PubMed] [Google Scholar]
- Franco AA, Buckwold S, Shin JW, Ascon M, Sears CL. Mutation of the zinc-binding metalloprotease motif affects Bacteroides fragilis toxin activity without affecting propeptide processing. Infect Immun. 2005;73:5273–5277. doi: 10.1128/IAI.73.8.5273-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Huet C, Sahuquillo-Merino C, Coudrier E, Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987;105:345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, Tomita K, Mimori T, Saya H. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- Jou T, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-kappa B plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001;123:421–427. doi: 10.1046/j.1365-2249.2001.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem. 2004;279:45519–45527. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414:555–558. doi: 10.1038/35107092. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, De Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Guggino WB, Montrose MH. Cellular differentiation regulates expression of Cl− transport and cystic fibrosis transmembrane conductance regulator mRNA in human intestinal cells. J Biol Chem. 1991;266:4495–4499. [PubMed] [Google Scholar]
- Moore WEC, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M, Tanaka S, Palacino J, Murayama O, Honda T, Sun X, Yasutake K, Nihonmatsu N, Wolozin B, Takashima A. Direct association of presenilin-1 with beta-catenin. FEBS Lett. 1998;433:73–77. doi: 10.1016/s0014-5793(98)00886-2. [DOI] [PubMed] [Google Scholar]
- Namavar F, Theunissen EB, Verweij-Van Vught AM, Peerbooms PG, Bal M, Hoitsma HF, MacLaren DM. Epidemiology of the Bacteroides fragilis group in the colonic flora in 10 patients with colonic cancer. J Med Microbiol. 1989;29:171–176. doi: 10.1099/00222615-29-3-171. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- Obiso RJ, Jr, Lyerly DM, Van Tassell RL, Wilkins TD. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect Immun. 1995;63:3820–3826. doi: 10.1128/iai.63.10.3820-3826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk BF, Kasper DL. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med. 1977;86:569–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerging Infect Dis. 2000;6:171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo MC, Arbo MD, Grindlinger J, Snydman DR. Attributable mortality of bacteremia associated with the Bacteroides fragilis group. Clin Infect Dis. 1995;20:1492–1496. doi: 10.1093/clinids/20.6.1492. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, De Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler M, Lotz M, Sears C, Pothoulakis C, Castagliuolo I, Wang CC, Sedivy R, Sogukoglu T, Cosentini E, Bischof G, et al. Bacteroides fragilis toxin 2 damages human colonic mucosa in vitro. Gut. 1999;44:504–510. doi: 10.1136/gut.44.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanfilippo L, Li CK, Seth R, Balwin TJ, Menozzi MG, Mahida YR. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-beta) by human colonic epithelial cells. Clin Exp Immunol. 2000;119:456–463. doi: 10.1046/j.1365-2249.2000.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, Gao H, Higgins MA, May PC, Ryan TP. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- Sears CL. The toxins of Bacteroides fragilis. Toxicon. 2001;39:1737–1746. doi: 10.1016/s0041-0101(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Sears CL, Myers LL, Lazenby A, Van Tassell RL. Enterotoxigenic Bacteroides fragilis. Clin Infect Dis. 1995;20(Suppl 2):S142–S148. doi: 10.1093/clinids/20.supplement_2.s142. [DOI] [PubMed] [Google Scholar]
- Sears CL, Buckwold SL, Shin JW, Franco AA. The C-terminal region of Bacteroides fragilis toxin is essential to its biological activity. Infect Immun. 2006;74:5595–5601. doi: 10.1128/IAI.00135-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med. 2005;11:496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Kopan R. Intramembrane proteolysis: theme and variations. Science. 2004;305:1119–1123. doi: 10.1126/science.1096187. [DOI] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA. 1998;95:14979–14984. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Dreyfus LA, Tzianabos AO, Hayashi C, Sears CL. Diversity of the metalloprotease toxin produced by enterotoxigenic Bacteroides fragilis. Infect Immun. 2002;70:2463–2471. doi: 10.1128/IAI.70.5.2463-2471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect Immun. 2004;72:5832–5839. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Shin J, Zhang G, Cohen M, Franco A, Sears CL. The Bacteroides fragilis toxin binds to a specific intestinal epithelial cell receptor. Infect Immun. 2006;74:5382–5390. doi: 10.1128/IAI.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Qian S, Soriano S, Wu Y, Fletcher AM, Wang XJ, Koo EH, Wu X, Zheng H. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10863–10868. doi: 10.1073/pnas.191284198. [DOI] [PMC free article] [PubMed] [Google Scholar]