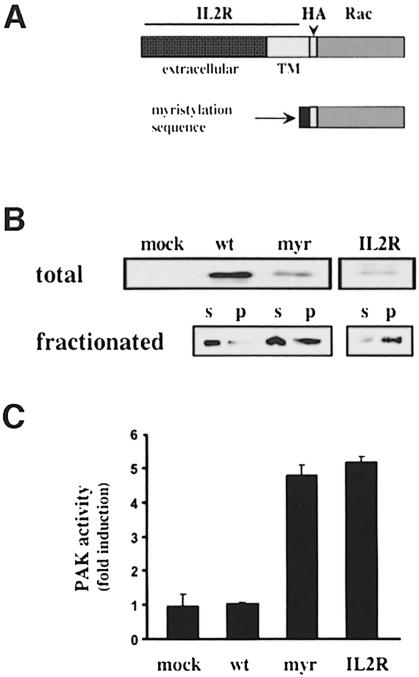
Fig. 6. Restoring PAK activity in suspended cells. (A) Diagram of membrane-targeted Rac mutants. HA-tagged wild-type Rac was fused to the transmembrane domain of the Tac subunit of the IL-2 receptor or the myristylation sequence from c-src. (B) Upper panel: a Western blot with anti-HA antibody of total cell lysate from transfected cells. The myristylated and IL2R–Rac chimeras were expressed at substantially lower levels than wild-type HA-Rac. Inclusion of cDNA for green fluorescent protein showed that transfection efficiency was ∼30%. Lower panel: the transfected Rac proteins in the soluble and particulate fractions of non-adherent cells subjected to homogenization and fractionation. Wild-type Rac was primarily cytosolic whereas both mutants show enhanced localization to the particulate fraction. (C) Serum-starved cells were placed in suspension for 3 h, stimulated with serum for 10 min and total endogenous PAK immunoprecipitated. PAK protein and kinase activity were determined and the specific activity calculated. Values are means ± SD (n = 4).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.