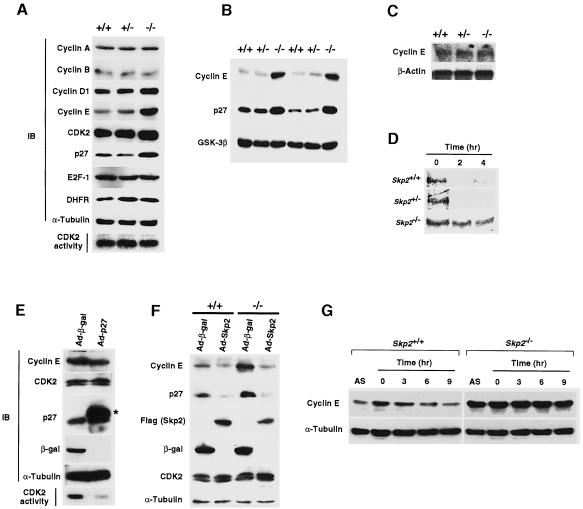
Fig. 4. Accumulation of cyclin E and loss of periodicity of cyclin E expression in Skp2–/– cells. (A) Immunoblot analysis (IB) of various cell cycle regulators in MEFs from Skp2+/+, Skp2+/– and Skp2–/– mice. An in vitro assay of CDK2 kinase activity is also shown. (B) Abundance of cyclin E and p27Kip1 in fetal liver from Skp2+/+, Skp2+/– and Skp2–/– mice. Immunoblot analysis of glycogen synthase kinase-3β (GSK-3β) is also shown as a control. (C) Northern blot analysis of cyclin E mRNA in MEFs. Polyadenylated RNA prepared from Skp2+/+, Skp2+/– and Skp2–/– MEFs was subjected to Northern blot analysis with mouse cyclin E cDNA or β-actin cDNA (control) probes. (D) Pulse–chase analysis of the turnover rate of 35S-labeled cyclin E in Skp2+/+, Skp2+/– and Skp2–/– MEFs. (E) Lack of effect of p27Kip1 overexpression on the abundance of cyclin E. Wild-type MEFs were infected with recombinant adenoviral vectors encoding either β-galactosidase (Ad-β-gal) or Flag epitope-tagged p27Kip1 (Ad-p27). Immunoblot analysis (IB) as well as an in vitro assay of CDK2 kinase activity were performed. The asterisk indicates the recombinant Flag-tagged p27Kip1. (F) Reversed expression levels of cyclin E and p27Kip1 by adenoviral transfer of the Skp2 gene into Skp2–/– MEFs. Cells were infected with recombinant adenoviral vectors encoding β-galactosidase (Ad-β-gal) or Flag-tagged Skp2 (Ad-Skp2). Cell lysates subsequently were prepared and subjected to immunoblot analysis with antibodies to cyclin E, p27Kip1, Flag (for Skp2), β-galactosidase, CDK2 or α-tubulin. (G) Impaired elimination of cyclin E during S–G2 phases in Skp2-deficient MEFs. Cell lysates prepared from MEFs in asynchronous culture (AS) or at the indicated times after release from aphidicolin-induced cell cycle arrest were subjected to immunoblot analysis with anti-cyclin E or anti-α-tubulin (control).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.