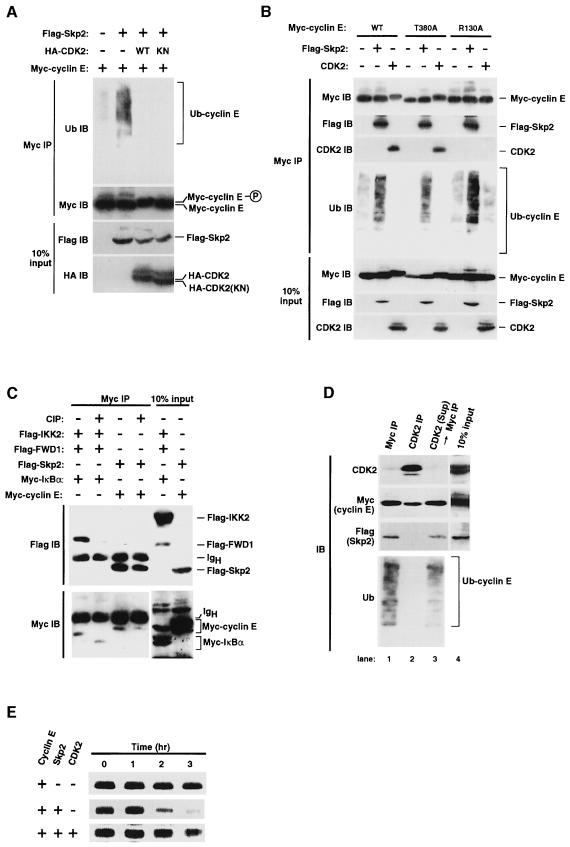
Fig. 6. Targeting of free cyclin E for Skp2-mediated ubiquitylation. (A) Effect of CDK2 on Skp2-mediated cyclin E ubiquitylation in vivo. Myc-tagged cyclin E either alone or together with Flag-tagged Skp2, HA-tagged wild-type (WT) CDK2 or an HA-tagged kinase-negative mutant (KN) of CDK2 were expressed in 293T cells. Proteins immunoprecipitated with anti-Myc were analyzed by immunoblotting with anti-Flag or anti-HA. Ten percent of the input for immunoprecipitation was also subjected to immunoblotting. (B) Interaction of the cyclin E mutants T380A and R130A with Skp2 and the effects of CDK2 on their ubiquitylation. Myc-tagged wild-type (WT) or mutant (T380A or R130A) cyclin E, Flag-Skp2 and HA-CDK2 were expressed in 293T cells. Proteins immunoprecipitated with anti-Myc were analyzed by immunoblotting with anti-Myc, anti-Flag, anti-CDK2 or anti-ubiquitin (this figure is overexposed compared with the others to show the basal level of cyclin E ubiquitylation). Ten percent of the input for immunoprecipitation was also subjected to immunoblotting. (C) Effect of phosphatase treatment on FWD1–IκBα and Skp2–cyclin E complexes. Myc-cyclin E, Myc-IκBα, Flag-Skp2, Flag-FWD1 and Flag-IKK2 (IκB kinase 2) were expressed in 293T cells. Proteins immunoprecipitated with anti-Myc were subjected (or not) to phosphatase (CIP) treatment and then to immunoblot analysis with anti-Flag or anti-Myc. Ten percent of the input for immunoprecipitation was also subjected to immunoblot analysis. (D) Sequential immunoprecipitation assay showing that only free cyclin E associates with Skp2 and undergoes ubiquitylation. 293T cells were transfected with expression plasmids encoding Myc-cyclin E and Flag-Skp2. Cell lysates were subjected to immunoprecipitation with anti-Myc (lane 1) or with anti-CDK2 (lane 2), and the resulting immunoprecipitates were subjected to immunoblot analysis with anti-CDK2, anti-Myc, anti-Flag or anti-ubiquitin. Cyclin E remaining in the supernatant (Sup) after immunoprecipitation with anti-CDK2 was then immunoprecipitated with anti-Myc and subjected to immunoblot analysis (lane 3). A portion of the cell lysate corresponding to 10% of the input for immunoprecipitation was also subjected to immunoblot analysis with anti-CDK2, anti-Myc or anti-Flag (lane 4). (E) Pulse–chase analysis of the effects of Skp2 and CDK2 on the turnover rate of cyclin E. Myc-cyclin E, Flag-Skp2 and CDK2 were expressed and radiolabeled in 293T cells. After incubation for the indicated times in the absence of isotope, radiolabeled proteins immunoprecipitated with the anti-Myc were analyzed by SDS–PAGE and autoradiography.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.