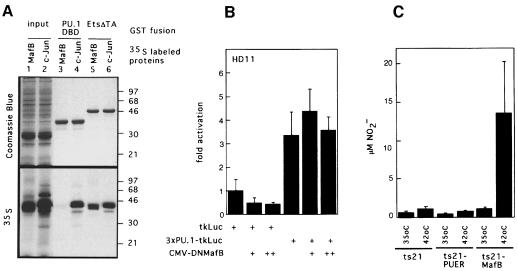
Fig. 12. Analysis of physical and functional interaction of PU.1 with MafB in macrophage differentiation. (A) Analysis of MafB interaction with Ets-1 and PU.1 in GST pull-down assays. In vitro translated [35S]methionine-labeled MafB (lane 1) or c-Jun (lane 2) were incubated with affinity matrix-bound GST fusion proteins of the PU.1 DNA-binding domain (PU.1-DBD, lanes 3 and 4) or the C-terminal portion of Ets-1 including its DNA-binding domain (Ets-ΔTA, lanes 5 and 6), washed, resuspended in SDS sample buffer and analyzed by SDS–PAGE. Coomassie Blue staining and autoradiography of the same gel are shown. Molecular weight markers (in kDa) are indicated on the right. (B) Effect of DNMafB on PU.1 activity in HD11 macrophages. HD11 macrophages were co-transfected with 0.5 µg of a luciferase reporter construct containing three multimerized PU.1 response elements (3×PU.1-tkLuc), with no, 0.5 µg (+) or 1 µg (++) of DNMafB constructs as indicated. Luciferase activities are expressed as fold activation over activity of a reporter containing no response elements (tkLuc). The assay was performed in duplicate and normalized to β-galactosidase activity derived from a co-transfected LTR-lacZ vector. Bars indicate the SEM. Results are representative of three independent experiments. (C) NO production by temperature-shifted ts21 myeloblasts expressing PU.1 or MafB. Equal numbers of cells from ts21, ts21-PUER and ts21-MafB clones were incubated at 35 or 42°C in the presence of 1 µM β-estradiol and stimulated with LPS after 48 h. NO release was measured 18 h later as NO2– concentration in the medium. The average data from four clones each are shown, with error bars indicating the SEM.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.