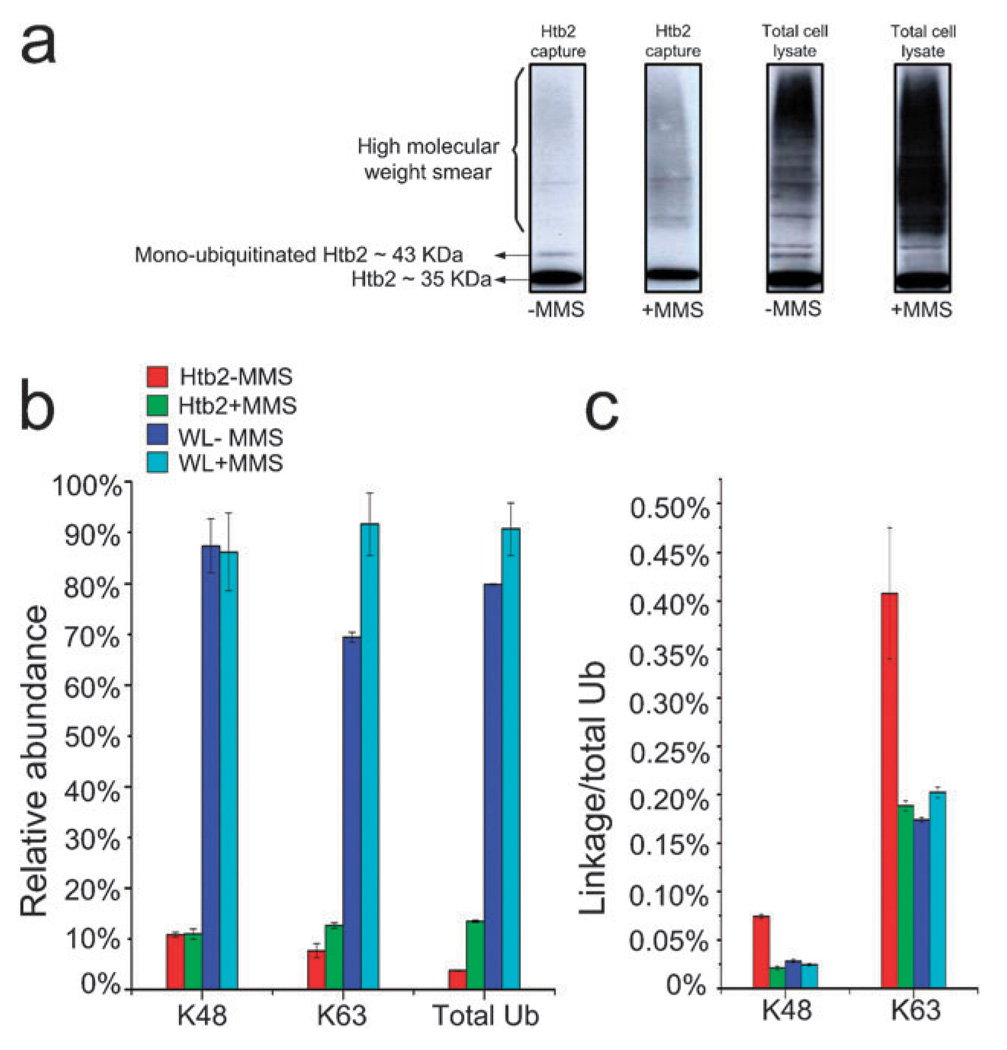
Fig. 4.
(a) Anti-Ub protein conjugate western blots for Htb2 protein (tagged with protein A which causes cross-reactivity with the Anti-Ub antibody) enriched from control and 0.1% MMS treated cells and their corresponding total cell lysates. The Htb2 blots suggest that the mono-ubiquitinated form of Htb2 disappears after the MMS treatment, while a high molecular weight smear appears after the MMS treatment. The total cell lysate immunoblots show a general increase in ubiquitination as a result of MMS treatment; (b) shows the average abundance of K48, K63 links and total Ub normalized to the highest value in each category. The data indicate that overall ubiquitination increases in both enriched Htb2 and total lysate after MMS treatment, whereas poly-ubiquitination via lysine 48 remains the same. An increase in the concentration of the K63 links suggests that additional poly-Ub conjugates in the Htb2 enriched sample are formed via lysine 63 as a result of MMS treatment; (c) the K48 and K63/total Ub ratio (representing poly-ubiquitination via K63) both show a decrease as a result of MMS treatment.