Abstract
AIM: To investigate the anti-inflammatory effects of cinnamon extract and elucidate its mechanisms for targeting the function of antigen presenting cells.
METHODS: Cinnamon extract was used to treat murine macrophage cell line (Raw 264.7), mouse primary antigen-presenting cells (APCs, MHCII+) and CD11c+ dendritic cells to analyze the effects of cinnamon extract on APC function. The mechanisms of action of cinnamon extract on APCs were investigated by analyzing cytokine production, and expression of MHC antigens and co-stimulatory molecules by quantitative real-time PCR and flow cytometry. In addition, the effect of cinnamon extract on antigen presentation capacity and APC-dependent T-cell differentiation were analyzed by [H3]-thymidine incorporation and cytokine analysis, respectively. To confirm the anti-inflammatory effects of cinnamon extract in vivo, cinnamon or PBS was orally administered to mice for 20 d followed by induction of experimental colitis with 2,4,6 trinitrobenzenesulfonic acid. The protective effects of cinnamon extract against experimental colitis were measured by checking clinical symptoms, histological analysis and cytokine expression profiles in inflamed tissue.
RESULTS: Treatment with cinnamon extract inhibited maturation of MHCII+ APCs or CD11c+ dendritic cells (DCs) by suppressing expression of co-stimulatory molecules (B7.1, B7.2, ICOS-L), MHCII and cyclooxygenase (COX)-2. Cinnamon extract induced regulatory DCs (rDCs) that produce low levels of pro-inflammatory cytokines [interleukin (IL)-1β, IL-6, IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-α] while expressing high levels of immunoregulatory cytokines (IL-10 and transforming growth factor-β). In addition, rDCs generated by cinnamon extract inhibited APC-dependent T-cell proliferation, and converted CD4+ T cells into IL-10high CD4+ T cells. Furthermore, oral administration of cinnamon extract inhibited development and progression of intestinal colitis by inhibiting expression of COX-2 and pro-inflammatory cytokines (IL-1β, IFN-γ and TNF-α), while enhancing IL-10 levels.
CONCLUSION: Our study suggests the potential of cinnamon extract as an anti-inflammatory agent by targeting the generation of regulatory APCs and IL-10+ regulatory T cells.
Keywords: Cinnamon extract, Inflammation, CD4 antigen, Antigen presenting cells, Cyclooxygenase-2, Tumor necrosis factor-α, Interleukin-10, Inflammatory bowel disease
INTRODUCTION
Oriental herbal medicines have been used for the treatment of various types of diseases for thousands of years[1,2]. Herbal medicines have immunomodulatory effects through diverse mechanisms of action. They can enhance immunity and induce tolerance in specific disease conditions[3,4]. For example, curcumin derived from Curcuma longa has potent anti-inflammatory activities by inhibiting nuclear factor-κB activation and blocking interleukin (IL)-12 signaling[5-7]. Andrographolide from Andrographis paniculata and herbkines have potent immunostimulatory activities. They increase lymphocyte proliferation, production of pro-inflammatory cytokines, and the humoral response[8-10].
Among many herbal medicines, Cinnamomum cassia bark is the outer skin of an evergreen tall tree belonging to the family Lauraceae. Cinnamon contains various active components including cinnamic aldehyde, cinnamyl aldehyde, tannin, mucus and carbohydrates. Previous studies have shown diverse biological functions of cinnamon extract such as antioxidant, antimicrobial and antidiabetic effects[11-16]. It also has potent anti-inflammatory properties by inhibiting the production of NO, cyclooxygenase (COX)-2 and prostaglandin (PG)E2 in macrophage cell lines[17,18]. However, it is still unclear how cinnamon modulates the function of immune cells, especially in vivo.
Antigen-presenting cells (APCs) process and present antigens on MHC molecules, and play pivotal roles in regulation of diverse immune responses. They can potentiate active immunity against pathogens while inducing immunotolerance to unharmful or self-antigens[19]. Recently, many studies have suggested the important role of APCs for induction of immunotolerance by inducing regulatory T cells and desensitization of effector T cells[20-22]. Regulatory APCs have potent therapeutic possibility to suppress various types of inflammatory immune disorders[20,23,24]. Among the APCs, regulatory dendritic cells (rDCs) have potent anti-inflammatory activities through diverse mechanisms of action. These include production of anti-inflammatory cytokines, induction of active cell death of effector T cells, inhibition of T-cell proliferation, and high level expression of indoleamine 2,3-dioxygenase (iDO)[20]. These immunomodulatory properties of rDCs induce regulatory T cells through cell to cell contact[23]. The potent anti-inflammatory properties of rDCs have been employed to treat several types of immune disorders including graft rejection, graft-versus-host disease and autoimmune disorders[24]. Hence, development of immunomodulators to enhance generation of rDCs could be a good strategy for the treatment of diverse inflammatory immune disorders.
In the present study, we evaluated the effects of cinnamon extract on modulation of effector function of APCs in vitro and in vivo. Treatment with cinnamon extract enhanced generation of rDCs that inhibited APC-dependent T-cell proliferation, and converted CD4+ T cells into IL-10high CD4+ T cells. Moreover, oral administration of cinnamon extract significantly suppressed experimental colitis by inhibiting expression of pro-inflammatory cytokines while enhancing IL-10 levels.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (6-8 wk old) and Do11.10 mice were purchased from SLC (Japan) or Jackson Laboratory (Bar Harbor, ME, USA), respectively. They were maintained under specific pathogen-free conditions in an animal facility at the Gwangju Institute of Science and Technology (GIST). All of the animal experiments were approved by the GIST Animal Care and Use Committee.
Preparation of cinnamon extract
Dried Cinnamomum cassia bark (Hwajin Distribution Co., Seoul, Korea) was pulverized and extracted in hot water for 3 h in a hot water extractor. The extract was filtered and the supernatant was concentrated with a rotary evaporator. The extract was then freeze-dried, resulting in a powder extract. The powder extract was suspended in sterilized distilled water at the appropriate concentrations. As we reported in our previous work[25], HPLC analysis was performed by comparing the levels of trans-cinnamic acid (Sigma, St Louis, MO, USA) and cinnamic aldehyde (kindly provided by Dr. Ehren, Germany) as known standard markers for the quality control of composition of cinnamon extract in each experiment[25]. Chromatography was carried out using 1% acetic acid (H2O)/methanol (50:50 v/v) at room temperature on a Phenomenex Luna 5u C18, 10-nm pore size, 250 × 4.60 mm I.D. column. The flow rate of the mobile phase was 2 mL/min. The amount of trans-cinnamic acid and cinnamic aldehyde was about 2.9 mg/g and 7.9 mg/g in each extract, respectively[25].
Cell lines
Raw 264.7 cells were obtained from the Korean Cell Line Bank (Seoul National University, Korea) and maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), 100 U/mL penicillin (Sigma) and 100 U/mL streptomycin (Sigma). Cells were cultured with 0.2 mg/mL cinnamon extract for 24 h and harvested for further analysis.
RNA isolation, cDNA synthesis, quantitative RT-PCR
Total RNA was prepared using TRI Reagent (Molecular Research Center) according to the manufacturer’s protocol. For reverse transcription, cDNA was generated using 1 μg total RNA, oligo(dT) primer (Promega, Madison, WI, USA) and Improm-II Reverse Transcriptase (Promega) in a total volume of 20 μL. One microliter of cDNA was amplified using the following RT-PCR primer sets: IL-1β (Forward 5'-GCAACTGTTCCTGAACTCAACT-3', Reverse 5'-ATCTTTTGGGGTCCGTCAACT-3'), IL-2 (Forward 5'-CCTGAGCAGGATGGAGAATTACA-3' and Reverse 5'-TCCAGAACATGCCGCAGAG-3'), IL-4 (Forward 5'-ACAGGAGAAGGGACGCCAT-3' and Reverse 5'-GAAGCCCTACAGACGAGCTCA-3'), IL-6 (Forward 5'-GAGGATACCACTCCCAACAGACC-3' and Reverse 5'-AAGTGCATCATCATCGTTGTTCA-3'), IL-10 (Forward 5'-ATAACTGCACCCACTTCCCA-3' and Reverse 5'-TCATTTCCGATAAGGCTTGG-3'), IL-12 p40 (Forward 5'-GGAAGCACGGCAGCAGAATA-3' and Reverse 5'-AACTTGAGGGAGAAGTAGGAATGG-3'), IL-17A (Forward 5'-TTCATCTGTGTCTCTGATGCT-3' and Reverse 5'-TTGACCTTCACATTCTGGAG-3'), interferon (IFN)-γ (Forward 5'-TCAAGTGGCATAGATGTGGAAGAA-3' and Reverse 5'-TGGCTCTGCAGGATTTTCATG-3'), tumor necrosis factor (TNF)-α (Forward 5'-CATCTTCTCAAAATTCGAGTGACAA-3' and Reverse 5'-TGGGAGTAGACAAGGTACAACCC-3'), transforming growth factor (TGF)-β (Forward 5'-GAAGGCAGAGTTCAGGGTCTT-3' and Reverse 5'-GGTTCCTGTCTTTGTGGTGAA-3'), HPRT (Forward 5'-TTATGGACAGGACTGAAAGAC-3' and Reverse 5'-GCTTTAATGTAATCCAGCAGGT-3'), B7.1 (Forward 5'-ACCCCCAACATAACTGAGTCT-3' and Reverse 5'-TTCCAACCAAGAGAAGCGAGG-3'), B7.2 (Forward 5'-TGTTTCCGTGGAGACGCAAG-3' and Reverse 5'-CAGCTCACTCAGGCTTATGTTTT-3'), B7-DC (Forward 5'-GTGCGATTTTGACCGCAGAG-3', Reverse 5'-CTAGGGATGTGGAACAAAGCC-3'), MHCII (Forward 5'-CACTCTCGTCTGTTCGGTGAC-3' and Reverse 5'-CCTCTCCCTGATGAGGGGTC-3'), ICOSL (Forward 5'-GACTGAAGTCGGTGCAATGGT-3' and Reverse 5'-TGGGTTTTCGATTTGCCAATAGA-3') and PD1L (Forward 5'-ATGCTGCCCTTCAGATCACAG-3' and Reverse 5'-TGGTTGATTTTGCGGTATGGG-3').
Immunoblotting
Proteins were resolved by 10% SDS-PAGE, transferred onto PVDF membranes (Bio-Rad) and subjected to Western blotting analysis using anti-Cox-2 (Cayman) and peroxidase-conjugated secondary antibodies (DAKO). Proteins were visualized with a chemiluminescence kit (Amersham Bioscience). β-tubulin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a loading control.
TNBS-induced colitis model
Induction of experimental inflammatory bowel disease (IBD) was carried out as described previously with minor modification[26]. To induce colitis, 1 mg TNBS in 50% ethanol was injected to slightly anesthetized mice through a catheter inserted into the rectum; 100 μL TNBS-ethanol mixture was carefully poured into the colon. The subsequent course of colitis was evaluated by assessing mortality, body weight decrease, and macroscopic and microscopic observations.
Macroscopic and histological evaluation of colitis
The intestines were removed from mice and evaluated as previously described (Marceau et al, 2004). For histological scoring, intestines were fixed with 4% paraformaldehyde in PBS and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin (HE) and scored as previously described[26]. To check the immunological changes, 5 d after IBD induction, mesenteric lymph nodes were obtained, suspended as single cells, and the expression level of cytokines and COX-2 were measure by quantitative real-time PCR or immunoblotting.
Isolation and activation of APCs and CD4+ T cells
For isolation of APCs (MHCII+ or CD11c+ DCs), spleens and lymph nodes isolated from C57BL/6 mice were ground with 30-μm pore meshes at the single cell level. Then, APCs purified with MHCII+ Isolation Kit (Miltenyi) or CD11c+ DC Isolation Kit (Miltenyi) were washed three times with ice-cold PBS and harvested for further analysis. CD4+ T cells from Do11.10 mice (Jackson Laboratory) were purified with CD4+ T cells Isolation Kit (Miltenyi).
Cell stimulation and treatment with cinnamon extract
Raw 264.7 cells were stimulated with lipopolysaccharide (LPS) (10 μg/mL) alone or in combination with cinnamon extract (0.2 mg/mL) for 24 h, and then washed three times with PBS. MHCII+ APCs or CD11c+ DCs were stimulated with LPS (10 μg/mL) alone or in combination with cinnamon extract (0.1, 0.3 and 0.5 mg/mL) for 24 h. After three washes, cells were harvested for further analysis.
Co-culture experiment
APCs (MHCII+ or CD11c+ DCs) were stimulated with LPS (10 μg/mL) alone or in combination with several doses of cinnamon extract (0.1, 0.3 and 0.5 mg/mL) for 24 h. The cells were incubated in mitomycin C (20 μg/mL) containing medium for 30 min at 37°C, and washed five times with ice-cold PBS. After 24 h stimulation, cells were co-cultured with CD4+ T cells obtained from Do11.10 mice in the absence or presence of ovalbumin peptide (Ova; 5, 20, 50 μg/mL) in 96-well plates for 56-72 h. After that, 0.5 μCi [H3]-thymidine (NEN) was added to each well and the cells were incubated for an additional 6 h. Cells were harvested and [H3]-thymidine uptake was measured by liquid scintillation counting. For checking functional change of co-cultured CD4+ T cells, CD4+ T cells were re-isolated from co-cultured population and mRNAs were obtained to analyze the levels of cytokine expression by quantitative RT-PCR.
Intracellular cytokine staining
To detect intracellular TNF-α and IL-10 positive populations, cells were stimulated with LPS alone or in combination with cinnamon extract for 24 h, fixed with 2% paraformaldehyde (Sigma) for 10 min, permeabilized with permeabilization buffer (0.5% saponin, 1% BSA in PBS) for 30 min, and stained with anti-TNF-α-PE (BD Pharmingen) or anti-IL-10-PE (BD Pharmingen) for 20 min at 4°C. IgG isotypes were used as a control for all flow cytometry analysis. The IgG-positive population was shown to be < 0.2% (data not shown).
Statistical analysis
A two-tailed Student’s t test was used, and P < 0.05 was considered to be statistically significant.
RESULTS
Treatment with cinnamon extract inhibits activation and maturation of APCs
Previous reports have shown that cinnamon components have a potent anti-inflammatory role by inhibiting NO synthesis[17,18] without providing any immunological evidence. In this study, we tested the anti-inflammatory efficacy of cinnamon extract and elucidated its working mechanisms by targeting APCs. To test the effect of cinnamon extract on activation and maturation of macrophages we used Raw 264.7 cells (Figure 1). Raw cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 24 h in the presence or absence of cinnamon extract at a concentration of 0.2 mg/mL, which did not induce any cell death or morphological change (data not shown). The expression levels of pro-inflammatory cytokines, co-stimulatory molecules and COX-2 were measured by real-time PCR. Raw cells upon activation produced a significant amount of IFN-γ and TNF-α. However, treatment with cinnamon extract significantly decreased expression levels of pro-inflammatory cytokine such as IL-1β and TNF-α (Figure 1A). Expression level of COX-2, a key inflammatory mediator[27], was also inhibited by cinnamon extract (Figure 1C). Next, to check the effect of cinnamon extract on maturation of Raw 264.7 cells, we analyzed the expression levels of activation markers for APCs such as MHCII and co-stimulatory molecules [B7.1, B7.2, ICOS ligand (ICOS-L) and PD1 ligand (PD-1L)] (Figure 1B). Consistent with inhibitory effects on pro-inflammatory cytokine expression, all the tested molecules were significantly reduced by treatment with cinnamon extract, except for PD-1L (Figure 1B). Since APCs can be activated by pathogen-associated molecular patterns[28], to mimic the in vivo situation, we stimulated Raw 264.7 cells with LPS, a TLR4 ligand (Figure 1C-E). Cinnamon extract was added during LPS stimulation and its effect on APC maturation and expression of pro-inflammatory molecules was analyzed. LPS treatment significantly increased expression levels of inflammatory cytokines, cell surface molecules and COX-2 compared to non-stimulated cells. However, cinnamon extract strongly inhibited expression of pro-inflammatory cytokines (IL-1β and TNF-α) (Figure 1C), co-stimulatory molecules (B7.1, B7.2, ICOS-L and MHCII) (Figure 1D) and COX-2 (Figure 1E). These results suggest that cinnamon extract has potent anti-inflammatory properties by inhibiting activation and maturation of APCs in vitro.
Figure 1.
Treatment of cinnamon extract inhibits maturation of macrophage cell line. Murine Raw 264.7 macrophages were stimulated with PMA/ionomycin in the absence or presence of 0.2 mg/mL cinnamon extract. Expression levels of cytokines (A) and co-stimulatory molecules (B) were measured by quantitative real-time PCR. To mimic in vivo stimulation, cells were treated with lipopolysaccharide alone or in combination with 0.2 mg/mL cinnamon extract. Expression level of cytokines (C), co-stimulatory molecules (D) and cyclooxygenase (COX)-2 (E) was measured by quantitative real-time PCR. Error bars indicated SD. aP < 0.05, bP < 0.005, cP < 0.001. Data are representative of three individual experiments.
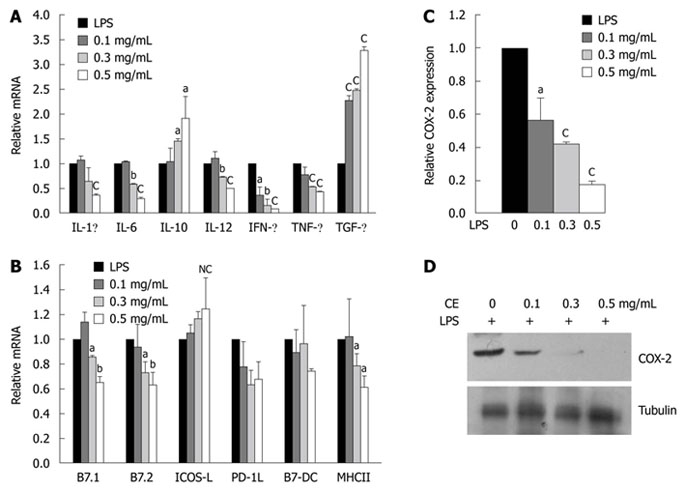
To test further the effect of cinnamon extract treatment on primary APCs, first, we titrated the dose of cinnamon extract in primary MHCII+ APCs that did not induce cytotoxic characteristics such as growth inhibition and apoptosis. Up to 0.7 mg/mL cinnamon extract did not show any cytotoxicity (data not shown). Next, primary MHCII+ APCs were stimulated with LPS alone or in combination with cinnamon extract (0.1, 0.3 and 0.5 mg/mL) for 24 h, and then the expression levels of cytokines, co-stimulatory molecules and COX-2 were measured by quantitative real-time PCR (Figure 2A-C). Treatment of cinnamon extract significantly decreased LPS-induced expression of pro-inflammatory cytokines (IL-1β, IL-6, IL-12, IFN-γ and TNF-α in a dose-dependent manner (Figure 2A). Cinnamon extract significantly upregulated the expression level of IL-10, an immunomodulatory cytokine, in a dose-dependent manner (Figure 2A). To confirm whether cinnamon extract also modulates protein level, we compared IL-10 and TNF-α, as a representative anti- or pro-inflammatory cytokine, respectively, by flow cytometry (Figure 2B). Consistent with mRNA data, treatment with cinnamon extract significantly decreased the TNF-α+ while increasing the IL-10+ population. LPS-stimulated cells produced high levels of TNF-α+ (81.8%), whereas treatment with 0.5 mg/mL cinnamon and LPS significantly reduced its production (40.9%) in a dose-dependent manner (Figure 2B). However, treatment with cinnamon extract significantly increased the IL-10+ population from 1.3% (LPS alone) to 8.36% (LPS with 0.5 mg/mL cinnamon extract) in a dose-dependent manner (Figure 2B). In addition, treatment with cinnamon extract significantly decreased COX-2 mRNA (Figure 2D) and protein (Figure 2E) levels in a dose-dependent manner. Next, we tested whether cinnamon treatment also modulated activation stage of primary MHCII+ APCs. MHCII+ APCs were stimulated with LPS alone or in combination with cinnamon extract, and then expression levels of co-stimulatory molecules were analyzed (Figure 2C). Cinnamon treatment significantly decreased MHCII molecules that play critical roles in T-cell activation. However, cinnamon treatment did not induce significant alteration in expression patterns of co-stimulatory molecules including B7.1, B7.2, ICOS-L and PD-1L (Figure 2D). Cinnamon treatment significantly increased expression levels of B7 DCs that have regulatory roles in inflammatory immune responses[29,30]. Hence, these data indicate that primary MHCII+ APCs upon treatment with cinnamon extract may gain immunoregulatory properties.
Figure 2.

Treatment with cinnamon extract inhibits maturation of MHCII+ APCs. MHCII+ APCs were stimulated with lipopolysaccharide (LPS) alone or in combination with cinnamon extract (0.1, 0.3 and 0.5 mg/mL). Expression levels of cytokines (A) and co-stimulatory molecules (C) were measured by quantitative real-time PCR. B: Intracellular expression levels of interleukin (IL)-10 and tumor necrosis factor (TNF)-α proteins were analyzed by flow cytometry. Effect of cinnamon extract treatment on cyclooxygenase (COX)-2 expression at the mRNA (D) and protein (E) level was measured by quantitative real-time PCR and immunoblotting, respectively. Error bars indicated SD. aP < 0.05, bP < 0.005, cP < 0.001. Data are representative of three individual experiments. IFN: Interferon.
Cinnamon extract treatment inhibits maturation of DCs
Based on the finding that cinnamon extract inhibits activation and maturation of MHCII+ APCs, we further tested whether treatment with cinnamon extract exerted a similar effect on DCs. CD11c+ DCs were isolated and stimulated with LPS alone or in combination with cinnamon extract for 24 h. Expression levels of cytokines, co-stimulatory molecules and COX-2 were measured by quantitative real-time PCR (Figure 3). CD11c+ DCs stimulated with LPS significantly increased expression levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12, IFN-γ and TNF-α. However, expression levels of pro-inflammatory cytokines were markedly reduced in a dose-dependent manner upon cinnamon extract treatment (Figure 3A). Cinnamon extract treatment significantly upregulated the expression levels of immunomodulatory cytokines such as IL-10 and TGF-β[31,32] in a dose-dependent manner (Figure 3A). Moreover, treatment with cinnamon extract significantly downregulated COX-2 mRNA (Figure 3C) and protein (Figure 3D) levels in a dose-dependent manner. Cinnamon extract treatment also downregulated the expression levels of B7.1, B7.2 and MHCII (Figure 3B). These data indicate that cinnamon extract inhibited maturation of CD11c+ DCs while inducing tolerogenic properties by increasing immunomodulatory cytokines.
Figure 3.
Treatment of cinnamon extract inhibits maturation of CD11+ dendritic cells. CD11+ dendritic cells (DCs) were stimulated in the absence or presence of cinnamon extract (CE) and expression levels of cytokines [interleukin (IL)-1β, IL-4, IL-6, IL-10, IL-12, interferon (IFN)-γ, tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β] (A) and co-stimulatory molecules (B7.1, B7.2, ICOS ligand, B7-DC and MHCII) (B) were measured by quantitative real-time PCR. Effect of cinnamon extract (CE) treatment on cyclooxygenase (COX)-2 expression at the RNA (C) and protein (D) level was measured by quantitative real-time PCR and immunoblotting, respectively. Error bars indicated SD. aP < 0.05, bP < 0.005, cP < 0.001. Data are representative of three independent experiments.
Cinnamon extract treatment inhibits APC-dependent T-cell proliferation
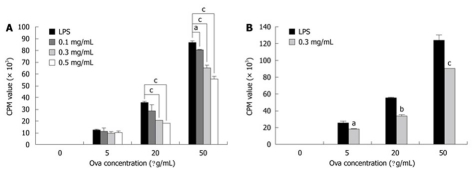
One of the major roles of APCs is to process and present antigens in the context of MHCII to T cells, which is a key event for induction of adaptive immune responses. We tested whether cinnamon extract treatment affected APC-dependent T-cell proliferation. Two types of primary APCs were isolated: MHCII+ APCs and CD11c+ DCs. They were pre-stimulated with LPS alone or in combination with cinnamon extract for 24 h, and then co-cultured with naïve T cells obtained from Do 11.10 mice that specifically expressed Ova-specific T-cell receptors. In the presence of Ova peptide, co-culture was continued for 72 h. T-cell proliferation was mainly dependent on antigen presenting and co-stimulatory capacity of APCs (MHCII+ or CD11c+). APCs (MHCII+ (Figure 4A) and CD11c+ DCs (Figure 4B) pre-treated with cinnamon extract significantly lowered APC-dependent T-cell proliferation in a dose-dependent manner (Figure 4A). These results suggest that treatment with cinnamon extract negatively modulated antigen presenting capacity of MHCII+ APCs or CD11c+ DCs to stimulate T-cell proliferation.
Figure 4.
Treatment with cinnamon extract inhibits APC-dependent T-cell proliferation. APC-dependent T-cell proliferation was measured. MHCII+ APCs (A) and CD11c+ dendritic cells (DCs) (B) were pre-pulsed in the absence or presence of cinnamon extract for 24 h. Then, they were co-cultured with CD4+ T cells isolated from Do11.10 mice in the presence of Ova peptide. After 72 h co-culture, T-cell proliferation was measured by [H3]-thymidine incorporation assay. Error bars indicated SD. aP < 0.05, bP < 0.005, cP < 0.001. Data are representative of three independent experiments.
DCs treated with cinnamon extract inhibit Th1 polarization while inducing IL-10high CD4 T cells
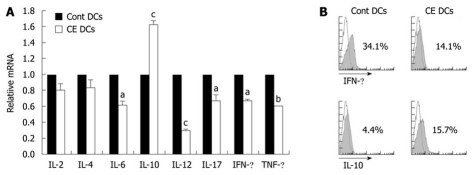
DCs play important roles in regulating the activity and differentiation of naïve T cells into diverse T cell lineages such as Th1, Th2 and Th17 types[33-35]. Since treatment with cinnamon lowered DC-dependent T-cell proliferation (Figure 4), we further tested the effects of cinnamon extract on DC-dependent T-cell polarization. T cells were co-cultured for 72 h with CD11c+ DCs pre-pulsed with LPS alone or in combination with 0.3 mg/mL cinnamon extract. Then, T cells were re-isolated and expression levels of T cell lineage-related cytokines were measured (Figure 5). T cells co-cultured with cinnamon-treated CD11c+ DCs produced much higher levels of IL-10 expression, while the levels of pro-inflammatory cytokines such as IL-6, IL-12, IL-17, IFN-γ and TNF-α were significantly decreased compared with control DCs (Figure 5A and B). No significant difference was observed in the levels of IL-2 and IL-4 between the treatment groups. These data indicate that CD11c+ DCs, upon treatment with cinnamon extract, could induce IL-10high CD4 T cells while inhibiting Th1 polarization.
Figure 5.
Cinnamon-extract-treated dendritic cells inhibit Th1 polarization. CD11c+ dendritic cells (DCs) were pre-pulsed with lipopolysaccharide (LPS) alone or in combination with cinnamon extract (CE) (0.2 mg/mL) for 24 h. Then, they were co-cultured with CD4+ T cells isolated from Do11.10 mice. A: After 72 h co-culture, CD4+ T cells were re-isolated from each treatment group and relative expression levels of cytokine mRNA were measured by quantitative real-time PCR; B: Intracellular protein levels of interleukin (IL)-10 and interferon (IFN)-γ in CD4+ T cells in each treatment group was analyzed by flow cytometry. Error bars indicated SD. aP < 0.05, bP < 0.005, cP < 0.001. Data are representative of three independent experiments.
Oral administration of cinnamon extract ameliorates experimental colitis
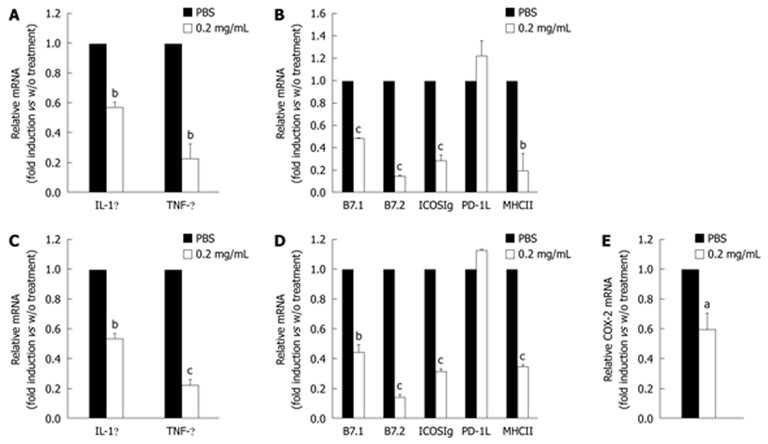
To confirm the anti-inflammatory efficacy of cinnamon extract in vivo, we tested whether oral administration of cinnamon extract could prevent progression of TNBS-induced experimental colitis, a typical Th1 type IBD[36]. Mice were fed orally with PBS alone or in combination with cinnamon extract (50 μg/g body weight) for 20 d, and experimental colitis was induced by intrarectal injection of TNBS. Clinical symptoms, weight loss and survival rate were monitored for 5 d. Both groups showed significant loss of body weight after 2 d of colitis induction. However, cinnamon-extract-treated mice began to recover weight loss starting from day 3, and almost restored their body weight at day 5, while PBS-treated mice continuously lost body weight during that period (Figure 6A). We also monitored survival rate. Cinnamon-extract-treated mice showed about 95% survival rate, while PBS-treated mice showed 50% survival on day 5 after colitis induction (data not shown). In accordance with weight loss and survival rate, colonic inflammation was significantly reduced in cinnamon-extract-treated mice compared with PBS-treated mice (Figure 6B). Tissue destruction and infiltration of mononuclear cells into the intestine, as shown by HE staining, were also significantly reduced by cinnamon extract (Figure 6C). To investigate further the underlying mechanism of cinnamon-extract-mediated IBD protection, we measured the levels of pro- or anti-inflammatory mediators between the treatment groups. First, we measured the mRNA and protein levels of COX-2, an inflammatory mediator that is highly expressed in inflamed tissues and activated immune cells. In accordance with in vitro results (Figure 2D and E), oral administration of cinnamon extract significantly reduced the expression levels of COX-2 in mesenteric lymph nodes at both mRNA and protein levels (Figure 6D). We also compared expression level of various pathogenic or protective cytokines such as IL-1β, IL-4, IL-6, IL-10, IFN-γ and TNF-α in cells isolated from mesenteric lymph nodes of cinnamon-extract-treated or non-treated mice. In agreement with clinical data, cells isolated from mesenteric lymph nodes of cinnamon-extract-treated mice expressed much lower levels of pathological cytokines such as IL-1β, IFN-γ and TNF-α (Figure 6E). No significant difference in the expression levels of IL-4 and IL-6 was observed between the treatment groups. Oral administration of cinnamon extract significantly upregulated IL-10 expression levels (Figure 6E). To confirm these data at the protein level, IL-10+ or TNF-α+ populations among total mesenteric lymph node cells were compared by flow cytometry. In accordance with mRNA level, oral administration of cinnamon extract significantly increased the IL-10+ population (PBS, 5.5% vs cinnamon extract, 14.7%) but decreased the TNF-α+ population (PBS, 7.3% vs cinnamon extract, 2.3%). These data suggest that the prophylactic effect of cinnamon extract against colitis pathogenesis is mediated by its anti-inflammatory properties.
Figure 6.
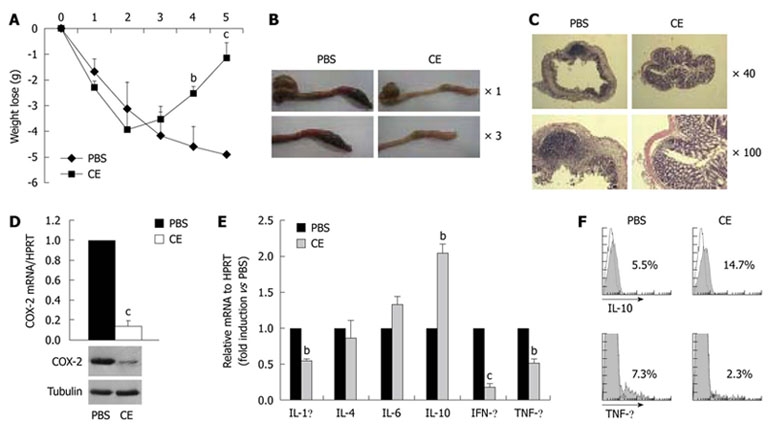
Oral administration of cinnamon extract ameliorates experimental colitis. Mice were orally fed with PBS or cinnamon extract (CE) (50 μg/g per day) for 20 d, and intestinal colitis was induced by intrarectal injection of TNBS (50 μg/g in 50% ethanol). A: A change in body weight was monitored for 5 d; B: Gross intestinal changes were analyzed with a magnifying glass 3 d after colitis induction; C: After HE staining, histological analysis was performed by comparing colon sections of treatment groups; D: After induction of inflammatory bowel disease, cyclooxygenase (COX)-2 expression in mesenteric lymph nodes was compared by quantitative real-time PCR and immunoblotting; E: Expression levels of cytokines in mesenteric lymphocytes were measured by quantitative real-time PCR; F: Intracellular protein levels of interleukin (IL)-10 and tumor necrosis factor (TNF)-α were measured by flow cytometry. Error bars indicated SD. bP < 0.005, cP < 0.001. Data are representative of three independent experiments. IFN: Interferon.
DISCUSSION
Treatment with cinnamon extract endowed APCs with tolerogenic characteristics resulting in inhibition of T-cell proliferation, T-cell polarization into Th1 type, while leading to IL-10high CD4 T cells. Furthermore, oral administration of cinnamon extract significantly suppressed the progression of experimental colitis by increasing IL-10 production while suppressing the levels of pro-inflammatory cytokines.
APCs express high levels of MHC molecules on their surface to present antigen to adaptive immune cells, especially T cells[19]. APCs are a heterogeneous population constituting various types of cells including DCs, B cells and macrophages[19]. APCs play important roles in the initiation and enhancement of immune responses, and for induction of immunotolerance as well[37-39]. DCs are key APCs that determine T-cell response and polarization into Th1 or Th2 cells[33]. APCs also play pivotal roles in maintenance of immunological homeostasis through various action mechanisms[20,24,40]. B cells have regulatory properties in modulating diverse inflammatory immune disorders such as IBD, collagen-induced arthritis, and autoimmune encephalomyelitis[24,41-45]. The regulatory properties of B cells are mainly mediated by production of anti-inflammatory cytokines such as IL-10 and TGF-β, which are important to generate regulatory T cells.
rDCs promote immunotolerance rather than immune activation[20,38,39]. rDCs usually express low levels of MHC molecules[23], co-stimulatory molecules and pro-inflammatory cytokines (IL-1β, IL-12 and TNF-α), while expressing high levels of IL-10, TGF-β and iDO. rDCs are resistant to maturation and help generation of regulatory T cells[23]. Their potent anti-inflammatory functions have been successfully demonstrated in various types of inflammatory immune disorders[23].
In this study, treatment with cinnamon extract significantly downregulated expression levels of MHCII and co-stimulatory molecules (B7.1 and B7.2) in macrophage cell lines (Figure 1D); primarily MCHII+ APCs (Figure 2C) and CD11c+ DCs (Figure 3B). Primary MCHII+ APCs significantly increased expression levels of B7-DC (PD-L2) upon cinnamon extract treatment in a dose-dependent manner (Figure 2C). B7-DC (PD-L2) is a ligand of PD1 which has potent anti-inflammatory properties to inhibit T-cell activation[46]. Hence, an increase of B7-DC expression by cinnamon extract may mediate its anti-inflammatory properties.
One of the properties of tolerogenic APCs is to express high levels of immune regulatory cytokines (IL-10 and TGF-β) and iDO, while expressing low levels of pro-inflammatory cytokines[23]. IL-10 and TGF-β are highly expressed in MCHII+ APCs, including B cells, macrophages and DCs, to suppress immune responses[47,48]. MHCII+ APCs upon cinnamon extract treatment produced significantly reduced levels of pro-inflammatory cytokines, including IL-1β, IL-6, IL-12, IFN-γ and TNF-α, while enhancing IL-10 levels (Figure 2A and B). Treatment with cinnamon extract also reduced NO production, a potent mediator of pro-inflammatory response in cinnamon-pulsed MCHII+ APCs (data not shown). In addition, cinnamon-pulsed DCs significantly decreased expression of pro-inflammatory cytokines, while upregulating IL-10 and TGF-β levels (Figure 3A). These results suggest that upregulated IL-10 and TGF-β levels in APCs by cinnamon extract may mediate downregulation of MHCII and co-stimulatory molecules[47,48].
Tolerogenic APCs inhibit effector T-cell function by suppressing T-cell proliferation and cytokine expression[23]. Upon LPS treatment, MHCII+ APCs and CD11c+ DCs strongly induced antigen-specific T-cell proliferation (Figure 4). However, cinnamon-pulsed MHCII+ APCs and CD11c+ DCs significantly reduced T-cell proliferation in response to antigen stimulation (Figure 4). Upon cinnamon extract treatment, how do APCs reduce their antigen-presenting capacity? An increased production of immunoregulatory cytokines (IL-10 and TGF-β) by cinnamon extract may reduce antigen-presenting capacity because increased levels of IL-10 and TGF-β suppress the expression of MHCII and co-stimulatory molecules on APC surfaces[47,48]. Another possibility is that cinnamon extract may modulate DC properties. Tolerogenic APCs could generate IL-10high (Tr1) or Foxp3+ regulatory T cells[23]. These latter cells have pivotal roles to suppress exaggerated immune responses in autoimmune, allergic and infectious diseases[49,50]. Here, we found that T cells cultured with cinnamon-pulsed DCs significantly decreased expression levels of pro-inflammatory cytokines such as IL-6, IL-12, IL-17, IFN-γ and TNF-α, while increasing IL-10 levels (Figure 5), which indicates that cinnamon-pulsed DCs inhibit Th1 polarization of naïve T cells rather than generate IL-10high regulatory T cells. Does cinnamon extract treatment increase Foxp3+ regulatory T cells? However, cinnamon extract treatment failed to increase Foxp3 expression within CD4+ T cells (data not shown).
COX-2 is a key mediator for PG synthesis. It promotes inflammatory immune response to exaggerate pathogenesis in rheumatoid arthritis, multiple sclerosis and cancer[51]. In the normal state, expression of COX-2 is almost undetectable. However, many cell types including chondrocytes, macrophages, DCs and epithelial cells express high levels of COX-2 by various stimuli such as IL-1β, TNF-α, phorbol esters, hypoxia, and LPS[51,52]. COX-2 plays a pivotal role in inflammation and tumor progression[53]. In our previous study, we have shown that cinnamon treatment effectively inhibits COX-2 expression in melanoma cell lines and in melanoma models[25]. In this study, we found that treatment with cinnamon extract also significantly inhibited COX-2 expression in Raw macrophage cells (Figure 1), primary MHCII+ APCs (Figure 2), CD11c+ DCs (Figure 3), and in cells isolated from mesenteric lymph nodes in experimental colitis (Figure 6). IL-10 and TGF-β downregulate COX-2 expression in the immune system[54,55], therefore, decreased expression of COX-2 levels (Figures 3, 4 and 6) may contribute to increased expression of IL-10 and TGF-β. However, we still do not know whether COX-2 inhibition by cinnamon is accomplished by intrinsic mechanisms such as active compounds of cinnamon, or extrinsic mechanisms mediated by immunomodulation. Currently, we are trying to identify active compounds from the cinnamon extract that have potent anti-COX-2 activities.
In summary, our study suggests a potent anti-inflammatory function of cinnamon extract that modulates effector function of APCs in vitro and in vivo. Cinnamon extract strongly inhibits maturation of APCs rather endows them with tolerogenic capacities that produce high levels of anti-inflammatory cytokines. Moreover, oral administration of cinnamon extract strongly ameliorated TNBS-induced experimental IBD by increasing IL-10 levels while downregulating pro-inflammatory cytokines. Further identification of the active components of cinnamon extract could lead to development of potent anti-inflammatory agents that target diverse inflammatory immune disorders.
COMMENTS
Background
Recently, the incidence of inflammatory immune disorders including inflammatory bowel disease, rheumatoid arthritis and allergic diseases has been growing fast. However, any conclusive medication for the treatment of these diseases is not available. Hence, oriental herbal medicines, which have been shown to have various bioactive components, are regarded as a potent source of medication for the development of immunomodulators.
Research frontiers
Cinnamomum cassia bark contains various bioactive molecules, and has been shown to have diverse biological functions such as antioxidant, antimicrobial and antidiabetic effects. However, although little evidence suggests its immunomodulatory properties such as inhibiting the production of NO, cyclooxygenase-2 and prostaglandin E2 in macrophage cell lines, it is still unclear how cinnamon modulates the function of immune cells, especially in vivo. In this study, the authors demonstrated potent anti-inflammatory properties of cinnamon through modulating the properties of antigen-presenting cells (APCs) in vitro and in vivo.
Innovations and breakthroughs
The work has demonstrated the potent anti-inflammatory properties of cinnamon extract. It modulates the properties of APCs to endow them with immunoregulatory phenotypes. In particular, cinnamon extract treatment of APCs affected their maturation, to have tolerogenic immune function by upregulating expression patterns of tolerogenic marker molecules such as B7-DC and cytokines including interleukin (IL)-10 and transforming growth factor-β. Moreover, cinnamon-extract-treated APCs affected effector functions of T cells by inhibiting their proliferation, while increasing IL-10 production, resulting in potent therapeutic effects in an animal model of intestinal colitis. The data strongly suggest that cinnamon extract is a potent anti-inflammatory herbal medicine that can be used to treat inflammatory bowel disease.
Applications
The authors have provided scientific evidence that cinnamon extract has potent anti-inflammatory properties. Further identification of its active components could lead to development of potent anti-inflammatory agents that target diverse inflammatory immune disorders.
Terminology
Cinnamon is from Cinnamomum cassia bark, which is a small evergreen tree belonging to the family Lauraceae. APCs are cells that display foreign antigen complexes with the MHC on their surface, for the recognition of this complex by T-cells, using the T-cell receptors.
Peer review
This is an interesting study that showed the anti-inflammatory effects of cinnamon extract. The authors demonstrated that cinnamon extract acts on APCs and IL-10+ regulatory cells. Cinnamon administration ameliorated experimental colitis by inhibiting inflammatory cytokines.
Footnotes
Supported by Grants from the BioGreen 21 Program, Rural Development Administration (PJ007054), Regional Technology Innovation Program of the MOCIE (RTI05-01-01) and by Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (A080588-20)
Peer reviewer: Joan Roselló-Catafau, PhD, Experimental Hepatology, Instituto de investigaciones Biomédicas de Barcelona, Consejo superior de Investigaciones Científicas, Rosselló 161, 080036-Barcelona, Spain
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
References
- 1.Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evid Based Complement Alternat Med. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calapai G, Caputi AP. Herbal medicines: can we do without pharmacologist? Evid Based Complement Alternat Med. 2007;4:41–43. doi: 10.1093/ecam/nem095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vojdani A, Erde J. Regulatory T Cells, a Potent Immunoregulatory Target for CAM Researchers: Modulating Tumor Immunity, Autoimmunity and Alloreactive Immunity (III) Evid Based Complement Alternat Med. 2006;3:309–316. doi: 10.1093/ecam/nel047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: the ultimate antagonist (I) Evid Based Complement Alternat Med. 2006;3:25–30. doi: 10.1093/ecam/nek022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, Lee CM, Ahn SC, Park YC, Park YM. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168:6506–6513. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- 7.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glöckner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 8.Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol. 2004;92:291–295. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Hong SH, Jeong HJ, Chung HS, Kim HR, Chae HJ, Shin T, Seo Y, Kim HM. An herbal formula, Herbkines, enhances cytokines production from immune cells. J Ethnopharmacol. 2005;98:149–155. doi: 10.1016/j.jep.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol. 2003;3:147–158. doi: 10.1046/j.1359-4117.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 11.Wijesekera RO. Historical overview of the cinnamon industry. CRC Crit Rev Food Sci Nutr. 1978;10:1–30. doi: 10.1080/10408397809527243. [DOI] [PubMed] [Google Scholar]
- 12.Schoene NW, Kelly MA, Polansky MM, Anderson RA. Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. Cancer Lett. 2005;230:134–140. doi: 10.1016/j.canlet.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Matan N, Rimkeeree H, Mawson AJ, Chompreeda P, Haruthaithanasan V, Parker M. Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int J Food Microbiol. 2006;107:180–185. doi: 10.1016/j.ijfoodmicro.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Jeon SM, Park EM, Huh TL, Kwon OS, Lee MK, Choi MS. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J Med Food. 2003;6:183–191. doi: 10.1089/10966200360716599. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 17.Lin CT, Chen CJ, Lin TY, Tung JC, Wang SY. Anti-inflammation activity of fruit essential oil from Cinnamomum insularimontanum Hayata. Bioresour Technol. 2008;99:8783–8787. doi: 10.1016/j.biortech.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Tung YT, Chua MT, Wang SY, Chang ST. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol. 2008;99:3908–3913. doi: 10.1016/j.biortech.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Murphy KM, Travers P, Walport M. Immunobiology, 8th ed. New York: Garland Science; 2008. pp. 182–183. [Google Scholar]
- 20.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 21.Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 22.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 23.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 24.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HK, Jeon WK, Hwang JS, Lee CG, So JS, Park JA, Ko BS, Im SH. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8+ T cells. Cancer Lett. 2009;278:174–182. doi: 10.1016/j.canlet.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2:879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 28.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, Lott F, Sun N, Welcher AA, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci USA. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 31.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 32.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 33.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 34.Jankovic D, Sher A, Yap G. Th1/Th2 effector choice in parasitic infection: decision making by committee. Curr Opin Immunol. 2001;13:403–409. doi: 10.1016/s0952-7915(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 35.Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001;22:450–457. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 36.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 38.Powrie F, Maloy KJ. Immunology. Regulating the regulators. Science. 2003;299:1030–1031. doi: 10.1126/science.1082031. [DOI] [PubMed] [Google Scholar]
- 39.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 40.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 41.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 42.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 44.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 45.Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 47.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 48.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 50.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 52.Chen T, Guo J, Yang M, Han C, Zhang M, Chen W, Liu Q, Wang J, Cao X. Cyclosporin A impairs dendritic cell migration by regulating chemokine receptor expression and inhibiting cyclooxygenase-2 expression. Blood. 2004;103:413–421. doi: 10.1182/blood-2003-07-2412. [DOI] [PubMed] [Google Scholar]
- 53.Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003;4:605–615. doi: 10.1016/s1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- 54.Berg DJ, Zhang J, Lauricella DM, Moore SA. Il-10 is a central regulator of cyclooxygenase-2 expression and prostaglandin production. J Immunol. 2001;166:2674–2680. doi: 10.4049/jimmunol.166.4.2674. [DOI] [PubMed] [Google Scholar]
- 55.Reddy ST, Gilbert RS, Xie W, Luner S, Herschman HR. TGF-beta 1 inhibits both endotoxin-induced prostaglandin synthesis and expression of the TIS10/prostaglandin synthase 2 gene in murine macrophages. J Leukoc Biol. 1994;55:192–200. doi: 10.1002/jlb.55.2.192. [DOI] [PubMed] [Google Scholar]