Figure 2. Domain Organization of Human ADAR s.
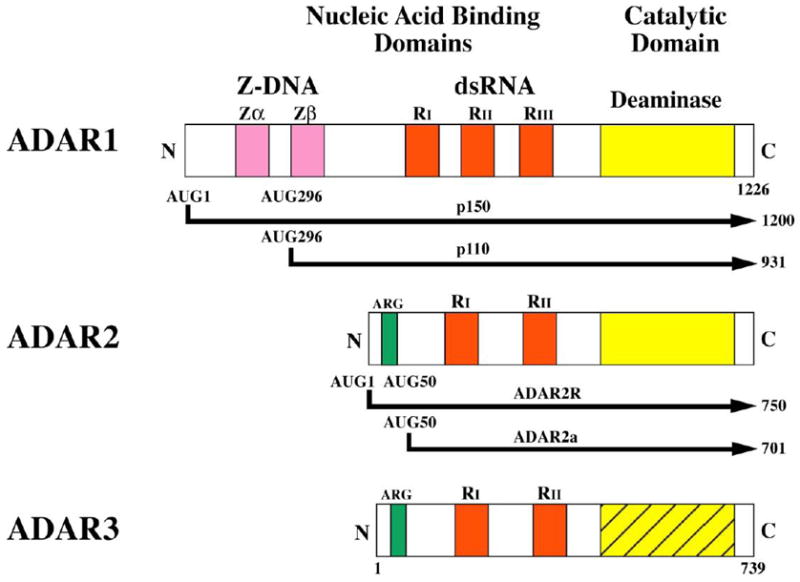
Three ADAR gene family members are known. ADAR1 and ADAR2 are active deaminase enzymes whereas ADAR3 is not known to possess deaminase activity. Alternative promoters and alternative splicing give rise to the different forms of ADAR1 and ADAR2. The p150 isoform of ADAR1 is IFN-inducible. The dsRNA binding domains (RI, RII, RIII), three present in ADAR1 p110 and p150 and two each in ADAR2 and ADAR3, are depicted in red. The C-terminal region deaminase catalytic domain is shown in yellow for ADAR1 and ADAR2, and the homologous region in stipled yellow for ADAR3 that is not yet demonstrated to be an active enzyme. The N-terminal region of the p150 form of ADAR1 possesses two Z-DNA binding domains (Zα and Zβ shown in pink, and the ADAR2R and ADAR3 proteins possess an arginine-rich domain (ARG) shown in green.
