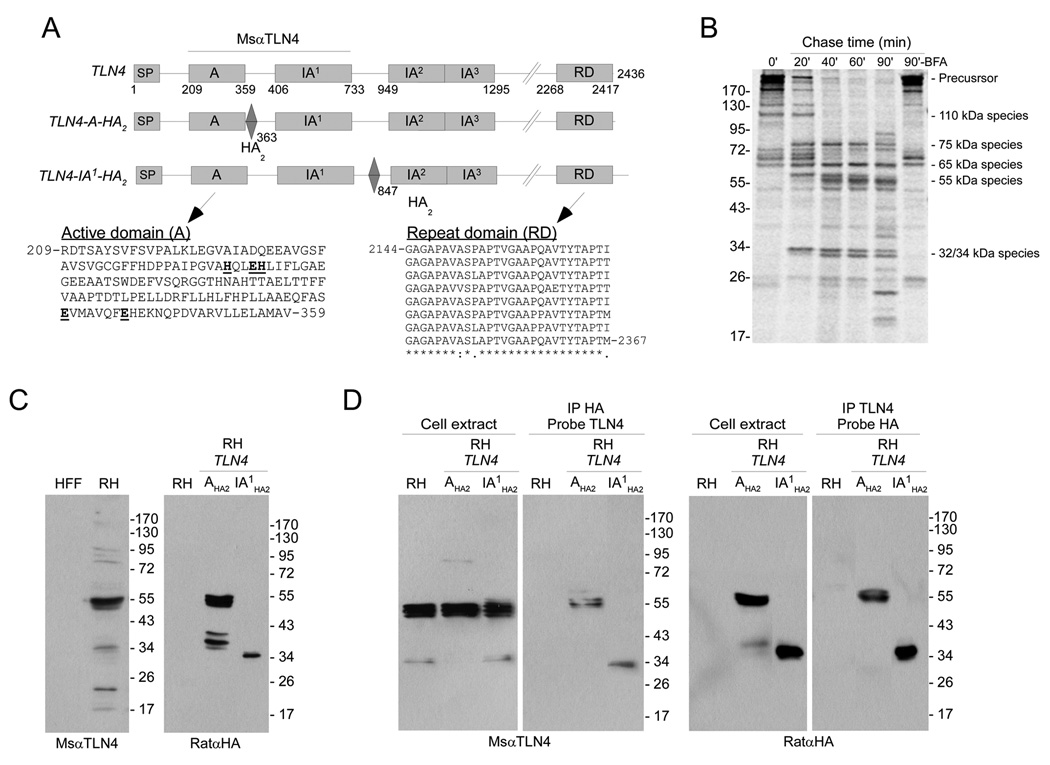
Figure 1.
TLN4 is extensively processed. (A) Schematic illustrations of TLN4 including domain features and placement of epitope tags. Amino acid positions of each domain are indicated underneath the TLN4 illustration and the positions of the HA2 epitope tag are indicated below the TLN4-A-HA2 and TLN4-IA1-HA2 illustrations. As demarked by a horizontal line, MsαTLN4 recognizes the active (A) and first inactive (IA1) domain of TLN4. Also shown are the sequences of the A domain and repeat domain (RD). The A domain active site residues in the HXXEHX69EX6E are emboldened and underlined. Note the 8 nearly perfect 28 aa repeats in the RD domain. Perfectly conserved positions (8 identical residues) are indicated with an asterisk, mostly conserved positions (6 or 7 identical residues) are denoted with a colon and partially conserved positions (5 or fewer identical residues) are marked with a period. (B) Pulse-chase immunoprecipitation analysis of TLN4 proteolytic processing. Tachyzoites were pulse labeled with 35S-Met and 35S-Cys for 15 min and chased with unlabeled amino acids for the indicated times. TLN4 fragments were immunoprecipitated with MsαTLN4 and analyzed by 7.5% SDS-PAGE and phosphorimaging. Positions of molecular weight standards (in kilodaltons, left) and the major TLN4 species (right) are marked. (C) Immunoblotting analysis of TLN4 products. The A and IA1 domains exist in a 55 kDa product based on recognition of untagged TLN4 by MsαTLN4 (left panel) and recognition of TLN4-A-HA2 by RatαHA (right panel, center lane). The linker region between IA1 and IA2 is a 34 kDa product based on recognition of TLN4-IA1-HA2 by RatαHA (right panel, right lane). (D) Reciprocal immunoprecipitation and immunoblotting analysis of TLN4 products. Shown are immunoblots of parasite cell extracts or immunoprecipitates with the indicated antibodies used for immunoprecipitation or probing.