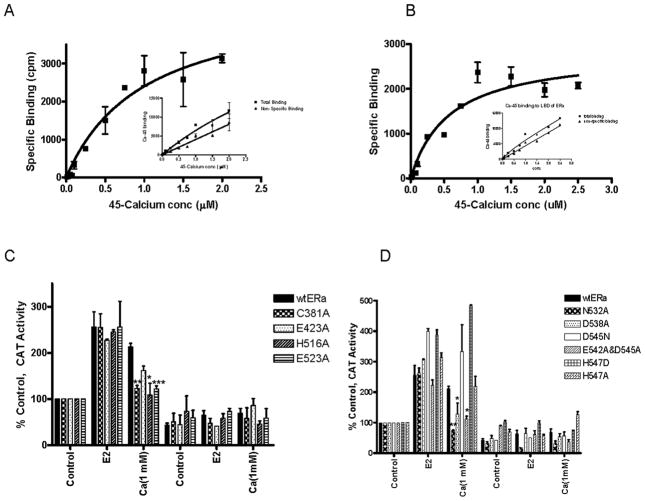
Figure 4. Calcium interaction with Estrogen Receptor-α.
A,B. Calcium binding to ERα. Purified recombinant ERα (A) or the purified recombinant LBD of ERα (B) was incubated overnight at 4°C with radioactive 45Ca2+ in the presence and absence of a 100-fold excess of calcium. Unbound 45Ca2+ was removed and specific binding was determined as total binding minus non-specific binding. The results were analyzed using nonlinear regression analysis. Representative graph of two or three independent experiments, respectively.
C. Calcium activation of ERα ligand binding domain mutants. COS-1 cells were transiently cotransfected with wtERα or C381A, E423A, H516A, E523A, N532A, D538A, D545N, H547A, H547D, and E542A/D545A, an estrogen responsive CAT reporter gene, andβ-gal and treated for 24 hours with estradiol (1nM), or calcium (1mM) with or without the antiestrogen ICI-182,780 (500nM). The amount of CAT activity was measured, normalized to the amount of β-galactosidase activity, and expressed as percent control. (mean±SD; three independent experiments). *, p < 0.05, **; p < 0.005; ***, p < 0.0005.