Table 2.
One pot synthesis of pyridines from α,β-unsaturated imines and alkynes
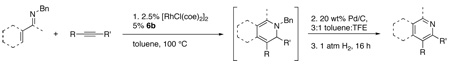 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Imine | Alkyne | Time (h) |
DHP | % Yielda (isolated) |
[O] Temp. (°C) |
[O] Time (h) |
Pyridine | Overall Yieldb |
| 1 | 2 | 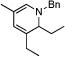 |
98 (97) | 0 | 8 | 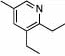 |
80% | ||
| 2 | 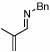 |
2 | 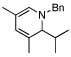 |
94 (99) | 0 | 8 | 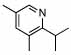 |
69% | |
| 3 | 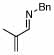 |
10 | 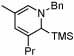 |
50 | 23 | 12 | 54% | ||
| 4 | 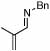 |
4 | 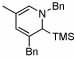 |
81 | 23 | 12 | 66% | ||
| 5 | 2 | 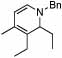 |
98 | 0 | 8 | 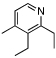 |
54% | ||
| 6 | 6 | 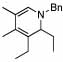 |
88 | 23 | 8 | 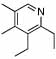 |
61% | ||
| 7 | 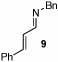 |
4 | 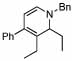 |
96 | 23 | 14 | 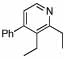 |
86% | |
| 8 | 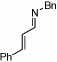 |
4 | 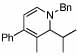 |
98 | 23 | 14 | 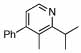 |
69% | |
| 9 | 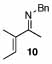 |
2 | 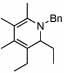 |
99 (78) | 75 | 16 | 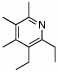 |
75% | |
| 10 | 2 | 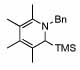 |
99 | 0 | 16 | 73% | |||
| 11 | 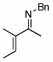 |
6 | 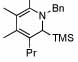 |
81 | 75 | 16 | 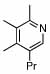 |
59% | |
| 12 | 8 | 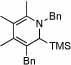 |
86 | 75 | 16 | 50% | |||
| 13 | 24 | 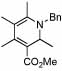 |
76 (70) | 120 | 16 | 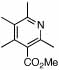 |
53%c | ||
| 14 | 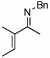 |
2 | 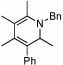 |
74 | 75 | 12 | 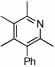 |
64%d | |
| 15 | 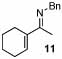 |
2 | 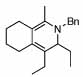 |
99 | 120 | 16 | 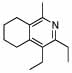 |
32% | |
Determined by NMR integration relative to 2,6-dimethoxytoluene as an internal standard.
Isolated overall yields based on starting α,β-unsaturated imine.
For this specific substrate, purified DHP was used for pyridine synthesis and the yield provided is the overall yield for the three steps.
The minor regioisomer with the Ph-substituent in the 2-position was isolated in 26% yield.
