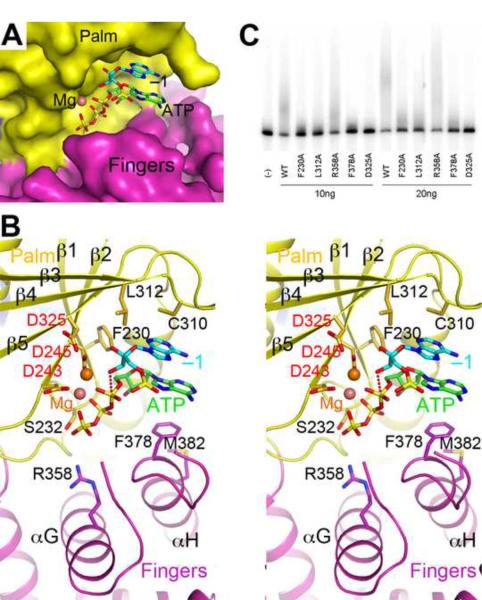
Figure 3.
Model of the active site of human PAPD1 with substrates. (A). Molecular surface of the active site region of PAPD1. The bound positions of Mg2+ (pink sphere), ATP (green) and the last nucleotide of the RNA substrate (cyan, labeled −1) are modeled from the structure of yeast PAP (Balbo and Bohm, 2007). (B). Stereo drawing of the active site model of human PAPD1. Side chains with potentially important roles in catalysis and/or substrate binding are shown as stick models and labeled. The side chain of Asp325 and the two Mg2+ ions are modeled. The inline nucleophilic attack of the 3' hydroxyl group of the last nucleotide of the RNA substrate (the −1 nucleotide) on the α-phosphate of ATP, modeled based on the structure of yeast PAP (Balbo and Bohm, 2007), is indicated by the dashed line in red. (C). Poly(A) polymerase assays for active site mutants of PAPD1. 10 or 20 ng of each enzyme was used in the assay, and the D325A mutant was included as a control.