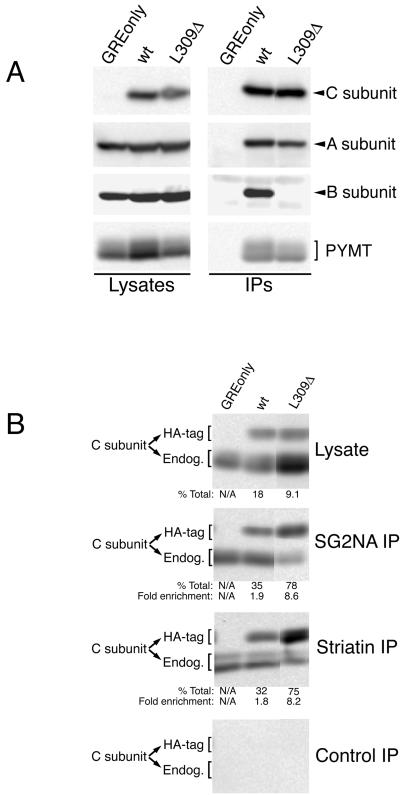
Figure 1.
Deletion of leucine 309 abolishes Bα subunit binding but not binding of MT, SG2NA, or striatin. (A) Lysates of NIH3T3 cells stably expressing MT antigen (PYMT) and HA-tagged wt C subunit, HA-tagged L309Δ C subunit, or empty vector (GREonly) were used to prepare anti-HA tag immunoprecipitates. Lysates and immunoprecipitates (IPs) were analyzed on the same 10% SDS-polyacrylamide gel and probed sequentially for epitope-tagged C subunit, A subunit, B subunit, and MT. The light bands above and below the position of B subunit in the immunoprecipitate lanes are antibody background bands. (B) Lysates of the same cell lines used in A were used to prepare SG2NA, striatin, and control immunoprecipitates (IPs) that were analyzed along with lysates by 10% SDS-PAGE and transferred to nitrocellulose. The membrane was probed with an anti-C subunit antibody (Transduction Laboratories) that recognizes both HA-tagged (HA-tag) and endogenous (Endog.) C subunits. Each band was quantitated using a Bio-Rad Fluor-S Max chemilumimager, which has a linear range of almost 5 orders of magnitude. The percentage of total C subunit that HA-tagged wt or L309Δ C subunit represent in lysates and in immunoprecipitates was calculated (% Total) and is shown beneath the respective panels. The ratio of % total in the immunoprecipitates to the % total in the respective lysates was calculated for each cell line as a measure of the efficiency of C subunit association with striatin or SG2NA and is indicated (Fold enrichment). The SDs for the fold enrichment values for SG2NA IPs were 0.4 and 1.7 for wt and L309Δ, respectively. For striatin IPs SDs were 0.4 and 1.1 for wt and L309Δ, respectively. All lanes in each panel were analyzed on the same gel but were not originally adjacent. The exposure times of the control, SG2NA, and striatin immunoprecipitate panels were equivalent. The C subunits migrate sometimes as singlets and sometimes as doublets; whether double or single bands are seen can vary for the same sample from gel to gel. This pattern of migration in SDS-PAGE has been noted previously for endogenous and epitope-tagged PP2A C subunits (Campbell et al., 1995; Turowski et al., 1995; Ogris et al., 1997) and does not appear to be due to degradation.