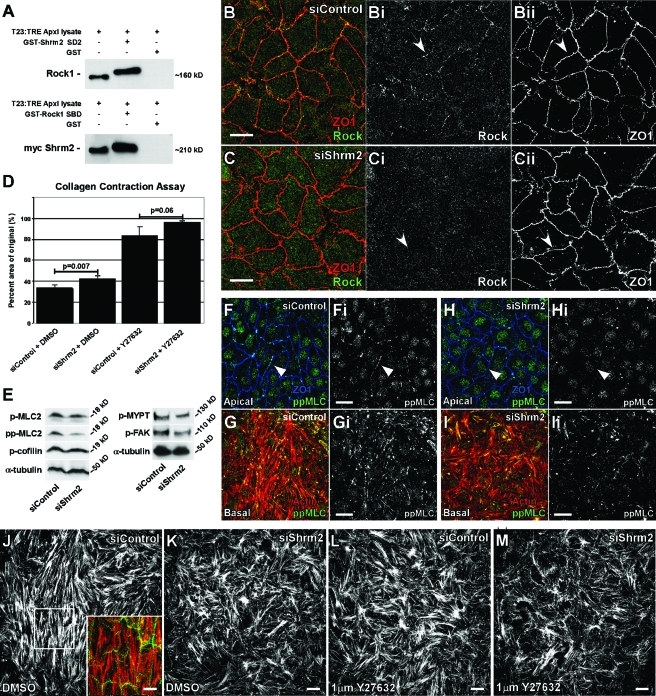
FIGURE 3:
Shrm2 interacts with Rock and promotes endothelial contractility. (A) A GST-tagged mShrm2 SD2 or a GST-tagged Shroom binding domain (SBD) of hRock1 were incubated with total cell lysate from T23 cells engineered to express myc-Shrm2 under the tetracycline response element promoter. Following GST pull down, the Shrm2 SD2 interacts with endogenous Rock1, and the Rock1 SBD interacts with full-length myc-Shrm2. (B and C) siControl (B) or siShrm2 (C) C166 cells were stained with Rock1 (Bi and Ci) and ZO1 (Bii and Cii) antibodies. Arrows indicate loss of Rock localization to tight junctions after Shrm2 knockdown. (D) Contractility of siControl or siShrm2 cells with or without the Rock inhibitor Y27632 was assessed through the ability of a monolayer to contract a collagen gel. Quantification is graphed as the percentage of area of the original after 4 h, represented by the mean ± SD (n = 3). (E) Phosphorylation of Rock effectors was visualized by Western blotting. α-Tubulin was used as a loading control. Representative blots from three independent experiments are shown. p-MLC2, phospho-myosin light chain 2 (Ser-19); pp-MLC2, diphospho-myosin light chain 2 (Thr-18/Ser-19); p-MYPT, phospho-myosin phosphatase binding subunit 1 (Thr-696); pFAK, phospho-focal adhesion kinase (Tyr-397). (F–I) Control (F and G) and Shrm2 knockdown (H and I) C166 cells were stained for ppMLC (Fi–Ii) and either ZO1 (F and H) or actin (G and I). Loss of Shrm2 leads to loss of pp-MLC2 at both stress fibers and cell–cell junctions (compare arrowheads). (J–M) Stress fiber organization was examined by immunostaining for actin in C166 cells treated with siControl (J), siShrm2 (K), siControl and Y27632 (L), and siShrm2 and Y27632 (M). Inset is a merge for apical Shrm2 (green) and basal stress fibers (red). Scale bars = 25 μm.