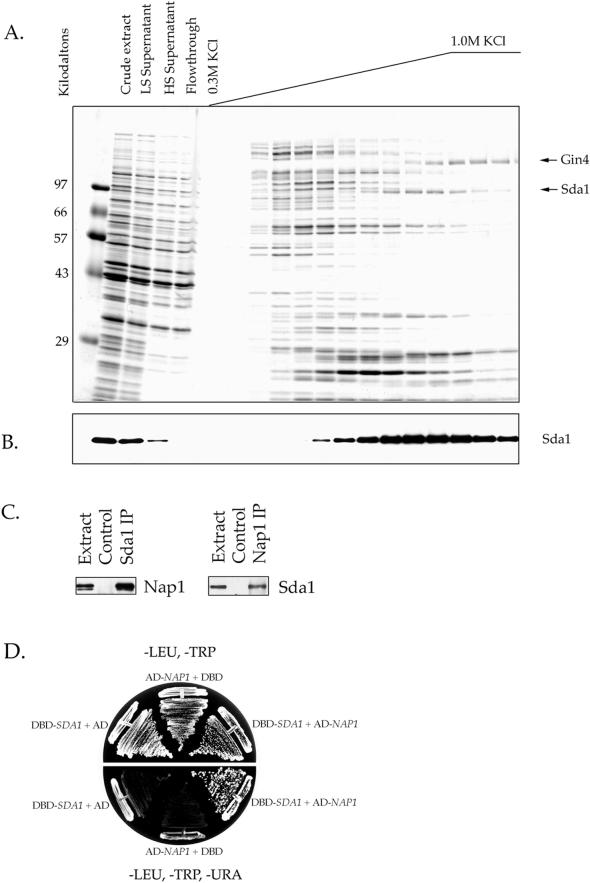
Figure 1.
Sda1 interacts with Nap1 in vitro and in vivo. (A) Affinity purification of Nap1-binding proteins. Crude extracts from rapidly growing yeast cells were loaded onto a Nap1 affinity column. After washing with buffer, the column was eluted with a KCl gradient. The samples from each fraction were TCA precipitated and loaded onto a 10% SDS-polyacrylamide gel. The gel is stained with Coomassie blue. The lane marked LS is a sample of the low-speed spin supernatant, whereas HS is the high speed supernatant. (B) The same fractions shown in A were probed by Western blotting with affinity-purified anti-Sda1 polyclonal antibody. The amount loaded onto the 10% SDS-polyacrylamide gel used for the Western blot in B was one-tenth the amount loaded in A. Note that the Sda1 protein is quantitatively depleted from the extract by the Nap1 affinity column. (C) Crude cell extracts were made from wild-type cells and used for Sda1 or Nap1 immunoprecipitations. The immunoprecipitations were washed in buffer containing 0.15 M NaCl. The Sda1 immunoprecipitation was probed with anti-Nap1 antibody to detect coprecipitated Nap1. The Nap1 immunoprecipitation was probed with anti-Sda1 antibody to detect coprecipitated Sda1. (D) Yeast two-hybrid analysis by using Sda1 cloned into the DBD vector pAS1 (marked with TRP1) as bait and Nap1 cloned into the transcriptional activating domain (AD) vector pACT (marked with LEU2) as prey. The interaction of Sda1 and Nap1 turns on the reporter genes URA3 and lacZ, which allows for growth on -URA media and the formation blue colonies on XGal media.