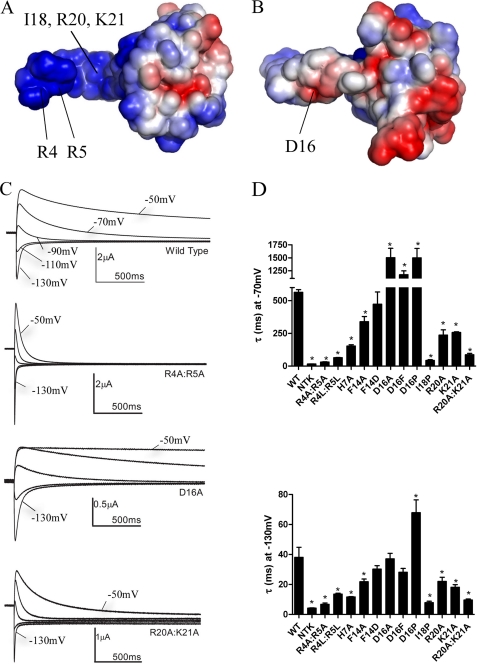
FIGURE 2.
A positively charged electrostatic surface on the NT1–26 domain is critical for normal deactivation. A, surface views of the EAG domain of hERG with the residues colored according to their electrostatic potential; areas of significant negative charge are shown in red and significant positive charge in blue and neutral in white. The NT1–26 domain extends out to the left of the molecule and is extensively positively charged on one side. B, 180° rotation of the structure in A about the horizontal axis. Labeled residues in A and B indicate those that when mutated significantly perturb deactivation gating. The position of the Ile18 residue is indicated with an arrow. Arg20 and Lys21 residues are located adjacent and to the right of Ile18 but are obscured in this orientation of the structure. C, representative current traces illustrating differences in rates of deactivation compared with WT hERG for channels in which charged NT1–26 residues have been mutated. Prepulses to +40 mV were applied before stepping down to a range of negative potentials. For clarity, only tail currents at potentials of −50 to −130 mV in 20-mV increments are shown. D, time constants for deactivation at −70 and −130 mV from single exponential fits of tail currents from NT1–26 mutants.* indicates time constants that are significantly different from WT hERG (p < 0.05, n ≥ 5).