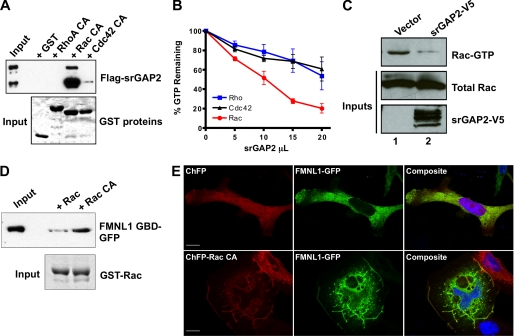
FIGURE 3.
Regulation of the Rac-srGAP2-FMNL1 pathway. A, shown is a GTPase specificity pulldown assay for srGAP2. Lysate from cells expressing srGAP2 (top panel, input) were incubated with GST-fused constitutively active Rho, Rac, or Cdc42 on glutathione beads (bottom panel, Coomassie stain). After centrifugation, bead fractions were assayed for bound srGAP2 by Western blot analysis (top panel). srGAP2 specifically associated with Rac but did not interact with Rho or Cdc42. B, shown is an in vitro GTPase assay for srGAP2 GAP specificity. 300 ng of purified Rho, Rac, and Cdc42 were loaded with radiolabeled GTP and incubated with increasing amounts of full-length srGAP2. srGAP2 exhibited greater GAP activity toward Rac when compared with Rho or Cdc42. C, shown is a cellular assay for srGAP2 Rac-GAP activity. Cells were transfected with empty vector or srGAP2 (bottom panel), and levels of Rac-GTP were analyzed (top panel) compared with total Rac (middle panel) by the p21-activated kinase pulldown assay. Cells expressing srGAP2 had lower levels of Rac-GTP, confirming Rac GAP activity in situ. D, shown is an activity-dependent interaction of FMNL1 with Rac. Lysates (Input) from cells expressing the FMNL1 GTPase binding domain (FMNL1 GBD; amino acids 1–450) were subjected to a pulldown assay using wild-type Rac or constitutively active Rac (RacCA) bound to beads as GST fusion (bottom panel). The FMNL1 GTPase binding domain preferentially interacted with active Rac. E, regulation of membrane targeting of FMNL1 by Rac is shown. Cells were co-transfected with either cherry fluorescent protein (ChFP) and FMNL1-GFP (top panels) or ChFP-Rac CA and FMNL1-GFP (bottom panels). Without active Rac, FMNL1 was predominately cytosolic, whereas with constitutively Rac, FMNL1 was enriched in membrane ruffles where it co-localized with Rac. The scale bar represents 15 μm.