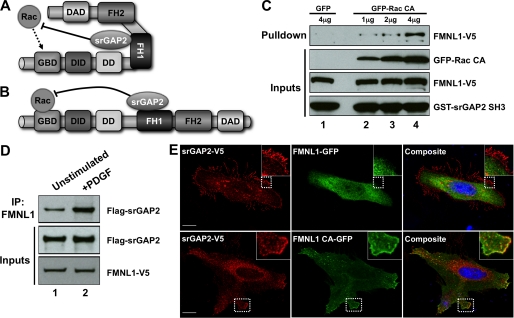
FIGURE 4.
Dynamic assembly of the srGAP2·FMNL1 complex. A and B, shown is a schematic of possible roles for srGAP2 in regulating GTPase signaling to FMNL1. A, in this model srGAP2 is bound to FMNL1 in the inactive state and limits the ability of Rac to activate FMNL1. This could favor the specificity of FMNL1 activation toward other GTPases such as Cdc42. B, in this model srGAP2 only binds to activated FMNL1, where it functions to turn off Rac after activation is achieved. C, GST srGAP2 SH3 (bottom panel) pulldown from cells expressing FMNL1 (second panel from bottom) and increasing amounts of constitutively active Rac (Rac CA; third panel from bottom; lanes 2–4). Whereas very little FMNL1 associated with the srGAP2 SH3 domain in the absence of active Rac (lane 1), increasing amounts of FMNL1 co-associated with the SH3 domain in the presence of increasing levels of active Rac (lanes 2–4). D, association of the srGAP2·FMNL1 complex was analyzed by co-immunoprecipitation without (IP, lane 1) or with (lane 2) PDGF stimulation from cells co-transfected with srGAP2 (middle panel) and FMNL1 (bottom panel). Increased complex association was observed following PDGF stimulation (top panel). E, srGAP2 preferentially associates with the active form of FMNL1 in cells. Cells were co-transfected with srGAP2 and either GFP- tagged wild-type FMNL1 (top panels) or GFP tagged constitutive active FMNL1 (bottom panels; FMNL1 CA). Immunostaining for srGAP2 showed cytosolic and membrane staining that co-localized with FMNL1 CA (bottom panels). The inset shows higher magnification images of the corresponding boxed regions. The scale bar represents 15 μm. DD stands for dimerization domain.