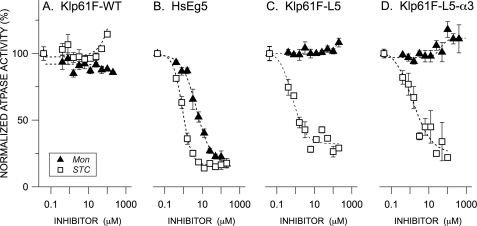
FIGURE 2.
Normalized rates of ATP hydrolysis for wild type HsEg5 and Drosophila melanogaster homolog Klp61F as a function of allosteric effector concentration. Basal ATPase rates (ADP/motor/sec) for wild type Drosophila Klp61F (A), HsEg5 (B), Klp61F-L5 (C), and Klp61F-L5-α3 (D) were measured in the presence of either STC (open squares) or monastrol (filled black triangles). The averages from 2–10 measurements and S.E. are normalized against the parent wild type kinesin motors. The monastrol inhibition curves exhibited by Klp61F-WT and both chimeras are indistinguishable (Wilcoxon signed rank test (α= 0.05)). In contrast, the STC inhibition curves exhibited by Klp61F-L5 and Klp61F-L5-α3 are indistinguishable from one another but are significantly different from that of Klp61F-WT (Wilcoxon signed rank test (α = 0.05).