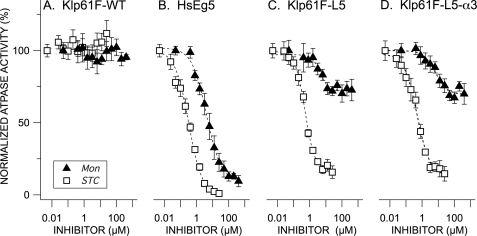
FIGURE 3.
Normalized MT-stimulated rates of ATP hydrolysis for wild type HsEg5 and D. melanogaster homologue Klp61F as a function of allosteric effector concentration. Steady-state, MT-ATPase rates (ADP/motor/s) for wild type Drosophila Klp61F (A), HsEg5 (B), Klp61F-L5 (C), and Klp61F-L5-α3 (D) were measured in the presence of either STC (open squares) or monastrol (filled black triangles). 4 μm taxol-stabilized microtubules were present in each assay. The averages of 3–10 measurements and S.E. are normalized against the parent kinesin motor, which has a rate of 100%. All the inhibition curves exhibited by the Klp61F chimeras are significantly different from the corresponding traces exhibited by Klp61F-WT (Wilcoxon signed rank test (α = 0.05).