Abstract
The anaerobic acetogenic bacterium Acetobacterium woodii employs a novel type of Na+-motive anaerobic respiration, caffeate respiration. However, this respiration is at the thermodynamic limit of energy conservation, and even worse, in the first step, caffeate is activated by caffeyl-CoA synthetase, which hydrolyzes ATP to AMP and pyrophosphate. Here, we have addressed whether or not the energy stored in the anhydride bond of pyrophosphate is conserved by A. woodii. Inverted membrane vesicles of A. woodii have a membrane-bound pyrophosphatase that catalyzes pyrophosphate hydrolysis at a rate of 70–120 milliunits/mg of protein. Pyrophosphatase activity was dependent on the divalent cation Mg2+. In addition, activity was strictly dependent on Na+ with a Km of 1.1 mm. Hydrolysis of pyrophosphate was accompanied by 22Na+ transport into the lumen of the inverted membrane vesicles. Inhibitor studies revealed that 22Na+ transport was primary and electrogenic. Next to the Na+-motive ferredoxin:NAD+ oxidoreductase (Fno or Rnf), the Na+-pyrophosphatase is the second primary Na+-translocating enzyme in A. woodii.
Keywords: Bacterial Metabolism, Bioenergetics, Electron Transport, Energy Metabolism, Membrane Enzymes, Acetobacterium, Na+ Gradient, Caffeate Respiration, Energy Conservation, Pyrophosphatase
Introduction
The anaerobic acetogenic bacterium Acetobacterium woodii is the prime example for an organism that has based its bioenergetics on Na+ instead of H+ (1–4). The energy-conserving pathways such as carbonate respiration (Wood-Ljungdahl pathway) (5, 6) are Na+-motive; the F1F0-ATP synthase uses Na+ as coupling ion (7, 8); and the flagellar motor is powered by an electrochemical sodium ion gradient (9). In addition, A. woodii is able to grow in the apparent absence of an electrochemical proton gradient, indicating that the growth-essential secondary transport systems are also driven by ΔμNa+.2
A. woodii not only grows by CO2 reduction, but it can also reduce the double bond of certain phenylacrylates, such as caffeate, in a process called caffeate respiration (3, 10–13), in which caffeate is reduced to hydrocaffeate, and a Na+ gradient is established across the cytoplasmic membrane that drives ATP synthesis (11). In this anaerobic respiration, oxidation of sugars or hydrogen yields reduced ferredoxin that is reoxidized by a membrane-bound Na+-translocating ferredoxin:NAD+ oxidoreductase (Fno/Rnf) (14, 15). This is the only coupling site known in caffeate respiration, and thermodynamic calculations show that this reaction allows for the transport of 1–2 mol of Na+. Assuming that 3–4 mol of Na+/mol of ATP are translocated, 3–4 mol of caffeate have to be reduced to synthesize 1 mol of ATP. Given this unfavorable energy balance, it was surprising to see that the electron acceptor caffeate is activated to caffeyl-CoA prior to its reduction by NADH via a hypothetical caffeyl-CoA reductase-Etf complex. Caffeate activation is catalyzed by an AMP-dependent caffeyl-CoA synthetase that hydrolyzes ATP to AMP and PPi (35). Thus, two energy-rich phosphate bonds have to be invested. Here, we have addressed whether or not some energy may be saved by conserving the energy of the PPi bond in the form of an electrochemical ion (Na+) gradient across the membrane. To search for such an activity, we have used an inverted membrane vesicle system of A. woodii, 22Na+, as a tracer and pyrophosphate as an energy source.
EXPERIMENTAL PROCEDURES
Growth of Cells and Preparation of Vesicles
A. woodii (DSM 1030) was grown under anaerobic conditions using 20 mm fructose as substrate as described (5, 7). The preparation of vesicles was done as described but slightly modified. For preparation of vesicles, the growth medium was supplemented with 420 mm sucrose and 8.1 mm MgSO4. 5 liters of medium were inoculated (4%), and the absorbance was followed at 600 nm. At A600 = 0.7–0.9, 70 μg of penicillin G/ml were added to the medium to induce protoplast formation. During further incubation, A. woodii formed protoplasts as monitored by microscopic observations. After 20 h, the culture consisted almost entirely of spherical forms that were highly sensitive to low osmolarity. These protoplasts were harvested aerobically by centrifugation (6250 × g, 20 min, 4 °C) and washed in 25 mm Pipes (sodium-free)/KOH buffer (pH 6.8) containing 25 mm MgSO4 and 420 mm sucrose. After washing the protoplasts, they were resuspended in a total volume of 300 ml and incubated with lysozyme (1 mg/ml) for 30 min at room temperature. The protoplasts were centrifuged (6250 × g, 20 min, 4 °C) and resuspended in 10–20 ml of Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4 and 420 mm sucrose. These protoplasts were passed through a French pressure cell at 41 megapascals and centrifuged three times (4500 × g, 35 min, 4 °C). The resulting supernatant (i.e. crude vesicles) was centrifuged further by ultracentrifugation (120,000 × g, 40 min, 4 °C). The pellet was washed in Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4 and 420 mm sucrose and centrifuged again. The resulting pellet was resuspended in the same buffer in a volume of 3 ml. Protein concentrations were determined by the method of Bradford (16).
PPi Hydrolysis
Pyrophosphatase (PPase)3 activity was determined by measuring the released phosphate using a method described by Heinonen and Lathi (17).
Measurement of Na+ Translocation
The experiments were performed under aerobic conditions in Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4 and 420 mm sucrose as described (7). The sodium and protein concentrations used are indicated. In a 1.5-ml Eppendorf cup, the vesicles, buffer, supplements (17 μm valinomycin, 1 mm N,N′-dicyclohexylcarbodiimide, NaCl, and 150 mm KCl), and 22NaCl (final activity of 0.5 μCi/ml) were combined and incubated at 30 °C for 120 min to assure equilibration of 22Na+ before the reaction was started with potassium pyrophosphate (final concentration of 1 mm or as indicated). 100 μm N,N,N,N′-tetracyclohexyl-1,2-phenylenedioxydiacetamide (ETH 2120) and 3,5-di-tert-butylhydroxybenzylidenemalonitrile (SF 6847) were added 6 min before the addition of PPi. 100-μl samples were withdrawn from the cup and passed over a column (0.5 × 3.2 cm) of Dowex 50-WX8 (100–200-mesh) (7). The vesicles were collected by washing the column with 1 ml of 420 mm sucrose. The radioactivity in the eluate was determined by liquid scintillation counting. For elucidating the effect of Mg2+ on the transport activity, the vesicles were prepared in the same buffer except that MgSO4 was omitted. MgSO4 and EDTA were added from stock solutions before the addition of 22NaCl.
Determination of Na+ Concentration
The Na+ concentration in the buffer was determined with an Orion 84-11 ROSS sodium electrode (Thermo Electron Corp., Witchford, UK) as described (18).
RESULTS
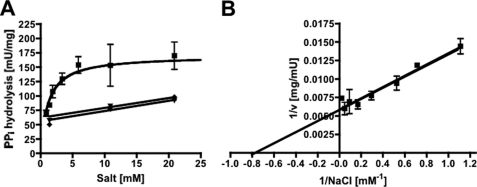
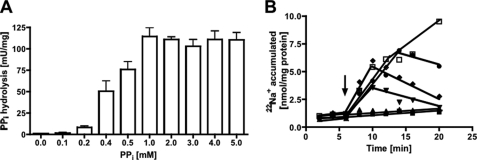
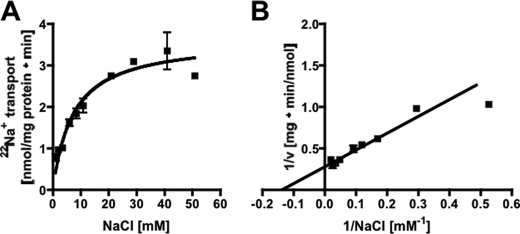
Inverted membrane vesicles (IMVs) of A. woodii prepared in Pipes/KOH buffer (pH 6.8) that also contained 25 mm MgSO4, 420 mm sucrose, and 1.9 mm NaCl were incubated at 30 °C. Upon the addition of PPi, it was hydrolyzed with a rate of 70–120 milliunits/mg (1 unit corresponding to 1 μmol of PPi/min at 1.9 mm NaCl). PPi hydrolysis was dependent on the external Na+ concentration. When no sodium was added to the assay, an activity of 68–74 milliunits/mg was measured (resulting from residual Na+ in the buffer and vesicles), but increasing Na+ concentrations led to increasing activities in a Michaelis-Menten-type fashion (Fig. 1A). The double-reciprocal plot is shown in Fig. 1B, and a Km of 1.1 ± 0.4 mm and a Vmax of 170 ± 12 milliunits/mg of protein were obtained. KCl and LiCl had only a marginal stimulatory effect. Pyrophosphate hydrolysis was dependent on the pyrophosphate concentration. Highest activities were obtained at 1 mm PPi (Fig. 2A). Pyrophosphate hydrolysis was accompanied by 22Na+ transport into the vesicles (Fig. 2B). As expected, 22Na+ transport increased with the pyrophosphate concentration. Also, at lower PPi concentrations, the Na+ gradient established collapsed after some time, which may be due to PPi depletion. There was a negligible activity at the lowest Na+ concentration achieved in the assay (900 μm, taking into account the buffer, vesicles, and potassium pyrophosphate), but transport activity increased with increasing Na+ concentration in a Michaelis-Menten fashion (Fig. 3A). The corresponding Lineweaver-Burk plot is shown in Fig. 3B. The Km for Na+ transport was determined to be 7.2 ± 1.4 mm, and the Vmax was 3.6 ± 0.25 nmol/min/mg of protein. This gives a Na+/PPi ratio of 0.02.
FIGURE 1.
Na+ dependence of PPi hydrolysis. A, PPi hydrolysis was determined in membrane vesicles in 25 mm Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4, 420 mm sucrose, and varying NaCl concentrations. The effect of NaCl (squares), KCl (diamonds), or LiCl (inverted triangles) on PPi hydrolysis. B, the corresponding Lineweaver-Burk plot for NaCl is shown. Data from two independent experiments are shown.
FIGURE 2.
Dependence of PPi hydrolysis and 22Na+ transport on the PPi concentration. Membrane vesicles (protein concentrations of 1.1 mg/ml in hydrolysis assay and 3.3 mg/ml in transport) in 25 mm Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4, 420 mm sucrose, and 1.9 mm NaCl showed PPi hydrolysis activity (A) and 22Na+ transport depending on the PPi concentration (B): 0 mm (closed squares), 0.1 mm (triangles), 0.5 mm (inverted triangles), 1 mm (diamonds), 2.5 mm (circles), 5 mm PPi (open squares). The arrow indicates the addition of PPi.
FIGURE 3.
Na+ dependence of PPi-dependent transport activity. Membrane vesicles (protein concentration of 3.6 mg/ml) were incubated in 25 mm Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4, 420 mm sucrose, and different NaCl concentrations. Transport rates were calculated from the initial slopes and plotted against the Na+ concentration in a Michaelis-Menten (A) or Lineweaver-Burk (B) plot. Data from two independent experiments are shown.
The intravesicular Na+ concentration in the IMVs (and thus the magnitude of the established Na+ gradient) was also dependent on the concentration of Na+. At 0.9 mm NaCl, a 10-fold accumulation was observed. Increasing Na+ concentrations led to decreasing Na+ gradients. At 1.9 mm NaCl, the accumulation factor was 9-fold, and at 40.9 mm NaCl, it was only 2.2-fold. Because the accumulation factor was higher at lower sodium concentrations, all subsequent experiments were performed in the presence of 1.9 mm NaCl.
It has been described previously that the PPase activity in Rhodospirillum rubrum and the activity of PPase isolated from mung beans are Mg2+-dependent (19, 20). To test the effect of Mg2+ on the PPi-dependent Na+ transport at IMVs of A. woodii, IMVs had to be prepared in Mg2+-free buffer. Unfortunately, when IMVs were prepared in the absence of MgSO4, the yield was very low, and the activity was very low, indicating that MgSO4 stabilizes the IMVs. When these IMVs were added to Mg2+-free assay buffer, Na+ transport (at 1.9 mm NaCl) was very low, with 0.004 nmol/min/mg of protein. The addition of Mg2+ to a final concentration of 25 mm stimulated Na+ transport by 2000% to 0.08 nmol/min/mg of protein. When the Mg2+-containing assay was supplemented with EDTA, Na+ transport dropped to 0.01 nmol/min/mg of protein. These data show that PPase-driven Na+ transport requires Mg2+.
If transport of 22Na+ is electrogenic, a membrane potential should be established that in turn should slow down 22Na+ transport. To test this, we used valinomycin in combination with KCl to dissipate the potential (ΔΨ). KCl itself (0, 50, 100, and 150 mm) had no effect on Na+ transport. However, in combination with valinomycin, 22Na+ transport was stimulated slightly (Fig. 4). This experiment indicates that PPi-driven 22Na+ transport is electrogenic.
FIGURE 4.
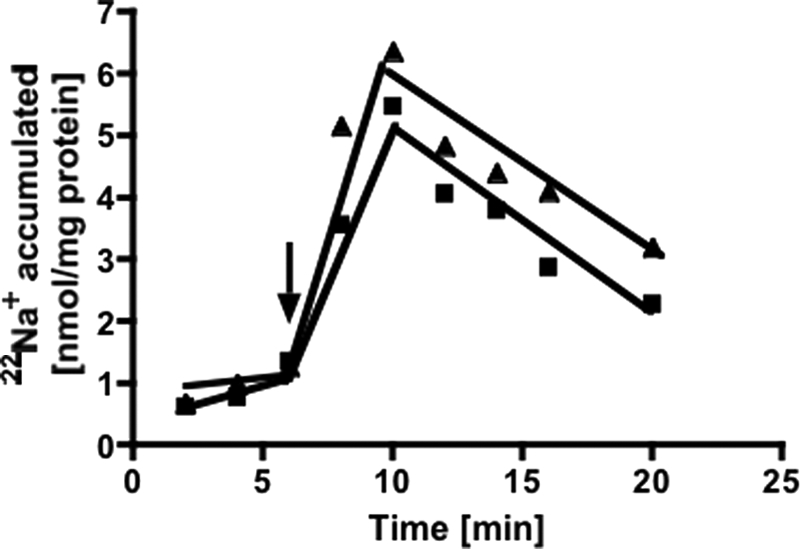
22Na+ transport is electrogenic. Membrane vesicles (protein concentration of 2.8 mg/ml) in Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4, 420 mm sucrose, 1.9 mm NaCl, 150 mm KCl, and 17 μm valinomycin showed 22Na+ transport upon addition of PPi (triangles) and no addition of valinomycin (squares). The arrow indicates the addition of PPi.
PPi-dependent Na+ transport should lead to the generation of a sodium ion potential that then could be used by the Na+ F1F0-ATP synthase of A. woodii to synthesize ATP. If this is true, inhibition of the Na+ F1F0-ATP synthase should lead to higher levels of 22Na+ in the lumen of the vesicles. This was indeed observed. In the presence of the F0-directed inhibitor N,N′-dicyclohexylcarbodiimide (1 mm, no extra sodium added to the assay), PPi-driven accumulation of 22Na+ was increased by 25%. However, the Na+ transport rate was not affected.
Pyrophosphatase activity could be directly or indirectly (via a primary H+ gradient in combination with a Na+/H+ antiporter) coupled to Na+ transport. To discriminate between these possibilities, we used protonophores or sodium ionophores and analyzed their effect on 22Na+ transport. The protonophore SF 6847 (100 μm) did not inhibit 22Na+ accumulation (Fig. 5). The effectiveness of the uncoupler was ensured by its ability to dissipate an artificial ΔpH created by a NH4+ diffusion potential (21). The sodium ionophore ETH 2120 (100 μm) completely inhibited 22Na+ accumulation; moreover, a previously established Na+ gradient was dissipated immediately upon the addition of ETH 2120 (Fig. 5). These data demonstrate that 22Na+ transport is directly linked to pyrophosphatase activity.
FIGURE 5.
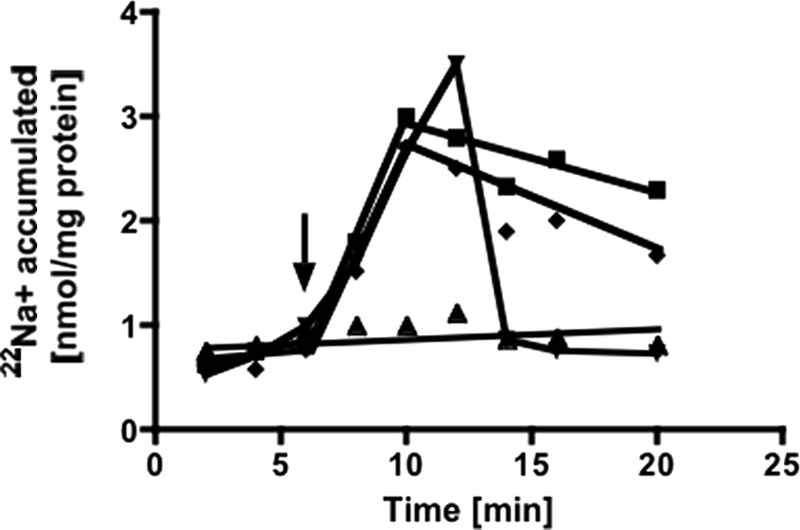
22Na+ transport is a primary event. Membrane vesicles (protein concentration of 2.8 mg/ml) in Pipes/KOH buffer (pH 6.8) containing 25 mm MgSO4, 420 mm sucrose, and 1.9 mm NaCl showed 22Na+ transport upon the addition of 1 mm PPi (squares). ETH 2120 (100 μm) was added 6 min before (triangles) or 6 min after (inverted triangles) addition of PPi. SF 6847 (100 μm) (diamonds) was added 6 min before the addition of PPi. The arrow indicates the addition of PPi.
DISCUSSION
Pyrophosphatases are commonly found enzymes. Next to soluble pyrophosphatases, membrane-bound enzymes have been found in bacteria, archaea, and plants (22–25). It has been shown that membrane-bound PPases couple PPi hydrolysis with proton or sodium ion transport (23, 26–29). Proton-pumping PPases acidify vacuoles in plants (22). In bacteria, they have been proposed to support energy conservation under conditions of energy deprivation (30, 31), and a light-induced maintenance of PPi levels by PPase has been suggested based on experiments with Rhodobacter capsulatus (32). PPi is a by-product of many biosynthetic processes for macromolecules, such as RNA and DNA synthesis, polysaccharide synthesis, formation of fatty acyl-CoA, and amino acid activation, and is formed in several reactions for substrate activation by anaerobic bacteria, e.g. activation of sulfate by sulfate-reducing bacteria or in activation of benzoate derivatives during anaerobic degradation of these compounds (23, 33).
Here, we have demonstrated a Na+-pumping pyrophosphatase at IMVs of A. woodii. The coupling efficiency was low, indicating leakiness of the vesicle system. Additionally, a second non-Na+-motive pyrophosphatase may contribute to PPi hydrolysis activity. This enzyme may be membrane-attached and thus also explain the different Km values for 22Na+ transport and PPi hydrolysis.
The overall ATP gain of caffeate respiration, as it is understood now, is very low. Electron flow from ferredoxin (−500 to −420 mV) to NAD+ (−320 mV) is accompanied with a free energy change of only −35 to −19 kJ/mol, and therefore, the Fno/Rnf complex allows for the synthesis of about one-third to one-half of an ATP. Thus, 2–3 mol of hydrogen have to be oxidized to get 1 ATP. Therefore, its highly economic for the cells to save the energy stored in PPi. The standard free energy stored in the PPi bond is equivalent to −22 kJ/mol. Assuming a membrane potential of −200 mV, this would allow for translocation of 1 Na+. Consequently, 3 mol of PPi have to be hydrolyzed for the production of 1 mol of ATP. This is only a small amount of energy saved, but rationalizing that caffeate respiration is at the thermodynamic limit, this adds another piece to the overall energy balance. Additionally, energy may be conserved by electron bifurcation from NADH to ferredoxin in the process of caffeyl-CoA reduction and the subsequent Fno/Rnf complex-catalyzed ferredoxin oxidation (34, 15). Furthermore, in the steady state of caffeate respiration, the activation of caffeate is likely to be catalyzed by an energy-saving CoA loop from hydrocaffeyl-CoA to caffeate (35).
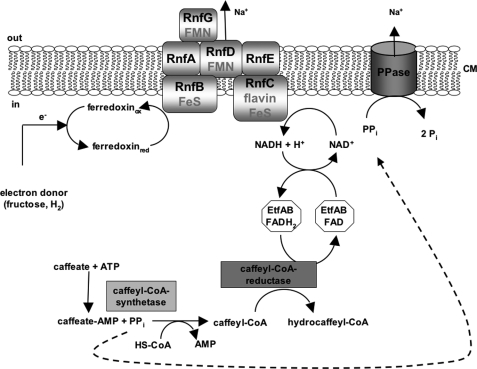
In summary, caffeate respiration involves an initial activation of the caffeate prior to its reduction by a AMP and PPi, forming caffeyl-CoA synthetase, yielding caffeyl-CoA. The produced PPi, as shown here, can be used by a membrane-bound pyrophosphatase to translocate Na+ across the cytoplasmic membrane in A. woodii. Therefore, the energy stored in the PPi bond is not lost but reinvested and conserved in a chemiosmotic Na+ potential (Fig. 6).
FIGURE 6.
Model of caffeate respiration in A. woodii. Flow of electrons from electron donors (fructose or hydrogen) to the acceptor caffeate is shown. The PPi generated by the caffeyl-CoA synthetase is used by the PPase to pump Na+ across the membrane.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 807).
V. Müller, unpublished data.
- PPase
- pyrophosphatase
- IMV
- inverted membrane vesicle.
REFERENCES
- 1. Müller V., Imkamp F., Rauwolf A., Küsel K., Drake H. L. (2004) in Strict and Facultative Anaerobes: Medical and Environmental Aspects (Nakano M. M., Zuber P. eds) pp. 251–281, Horizon Biosciences, Norfolk [Google Scholar]
- 2. Müller V. (2003) Appl. Environ. Microbiol. 69, 6345–6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt S., Biegel E., Müller V. (2009) Biochim. Biophys. Acta 1787, 691–696 [DOI] [PubMed] [Google Scholar]
- 4. Müller V., Aufurth S., Rahlfs S. (2001) Biochim. Biophys. Acta 1505, 108–120 [DOI] [PubMed] [Google Scholar]
- 5. Heise R., Müller V., Gottschalk G. (1989) J. Bacteriol. 171, 5473–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heise R., Müller V., Gottschalk G. (1993) FEMS Microbiol. Lett. 112, 261–267 [Google Scholar]
- 7. Heise R., Müller V., Gottschalk G. (1992) Eur. J. Biochem. 206, 553–557 [DOI] [PubMed] [Google Scholar]
- 8. Fritz M., Müller V. (2007) FEBS J. 274, 3421–3428 [DOI] [PubMed] [Google Scholar]
- 9. Müller V., Bowien S. (1995) Arch. Microbiol. 164, 363–369 [Google Scholar]
- 10. Imkamp F., Biegel E., Jayamani E., Buckel W., Müller V. (2007) J. Bacteriol. 189, 8145–8153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imkamp F., Müller V. (2002) J. Bacteriol. 184, 1947–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller V., Imkamp F., Biegel E., Schmidt S., Dilling S. (2008) Ann. N.Y. Acad. Sci. 1125, 137–146 [DOI] [PubMed] [Google Scholar]
- 13. Tschech A., Pfennig N. (1984) Arch. Microbiol. 137, 163–167 [Google Scholar]
- 14. Biegel E., Müller V. (2010) Proc. Natl. Acad. Sci. U.S.A., 107, 18138–18142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biegel E., Schmidt S., Gonzáles J. M., Müller V. (2011) Cell Mol. Life Sci. 10.1007/s00018-010-0555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 17. Heinonen J. K., Lahti R. J. (1981) Anal. Biochem. 113, 313–317 [DOI] [PubMed] [Google Scholar]
- 18. Pisa K. Y., Huber H., Thomm M., Müller V. (2007) FEBS J. 274, 3928–3938 [DOI] [PubMed] [Google Scholar]
- 19. Maeshima M., Yoshida S. (1989) J. Biol. Chem. 264, 20068–20073 [PubMed] [Google Scholar]
- 20. Sosa A., Ordaz H., Romero I., Celis H. (1992) Biochem. J. 283, 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura T., Hsu C., Rosen B. P. (1986) J. Biol. Chem. 261, 678–683 [PubMed] [Google Scholar]
- 22. Rea P. A., Poole R. J. (1993) Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 157–180 [Google Scholar]
- 23. Maeshima M. (2000) Biochim. Biophys. Acta 1465, 37–51 [DOI] [PubMed] [Google Scholar]
- 24. Serrano A., Perez-Castiñeira J. R., Baltscheffsky H., Baltscheffsky M. (2004) J. Bioenerg. Biomembr. 36, 127–133 [DOI] [PubMed] [Google Scholar]
- 25. Docampo R., de Souza W., Miranda K., Rohloff P., Moreno S. N. (2005) Nat. Rev. Microbiol. 3, 251–261 [DOI] [PubMed] [Google Scholar]
- 26. Belogurov G. A., Malinen A. M., Turkina M. V., Jalonen U., Rytkönen K., Baykov A. A., Lahti R. (2005) Biochemistry 44, 2088–2096 [DOI] [PubMed] [Google Scholar]
- 27. Malinen A. M., Belogurov G. A., Baykov A. A., Lahti R. (2007) Biochemistry 46, 8872–8878 [DOI] [PubMed] [Google Scholar]
- 28. Baltscheffsky M., Schultz A., Baltscheffsky H. (1999) FEBS Lett. 457, 527–533 [DOI] [PubMed] [Google Scholar]
- 29. Malinen A. M., Baykov A. A., Lahti R. (2008) Biochemistry 47, 13447–13454 [DOI] [PubMed] [Google Scholar]
- 30. Skulachev V. P. (1977) FEBS Lett. 74, 1–9 [DOI] [PubMed] [Google Scholar]
- 31. Nyren P., Strid A. (1991) FEMS Microbiol. Lett. 77, 265–269 [Google Scholar]
- 32. Nore B. F., Nyren P., Salih G. F., Strid A. (1990) Photosynth. Res. 24, 75–80 [DOI] [PubMed] [Google Scholar]
- 33. Schöcke L., Schink B. (1998) Eur. J. Biochem. 256, 589–594 [DOI] [PubMed] [Google Scholar]
- 34. Herrmann G., Jayamani E., Mai G., Buckel W. (2008) J. Bacteriol. 190, 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hess V., Vitt S., Müller V. (2011) J. Bacteriol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]