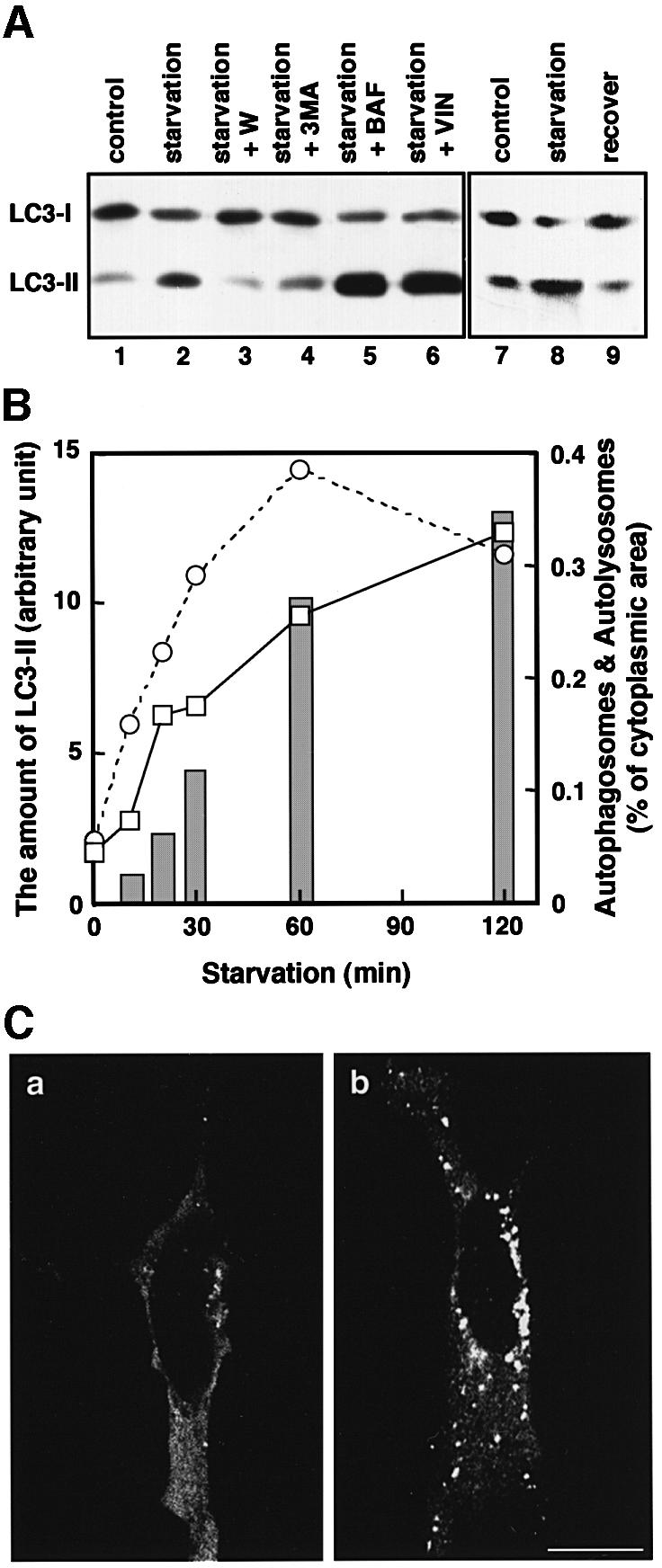
Fig. 7. The change in the amount of LC3-II corresponds to autophagosome formation. (A) HeLa cells were cultured at 37°C for 90 min in 10% FCS/DMEM (lane 1) or Hanks’ solution (lanes 2–6) containing the following reagents: 1% dimethylsulfoxide (control, lanes 1 and 2); 0.1 µM wortmannin (lane 3); 10 mM 3-methyladenine (lane 4); 0.1 µM bafilomycin A1 (lane 5); and 50 µM vinblastine (lane 6). In a separate experiment, HeLa cells were cultured at 37°C for 120 min in 10% FCS/DMEM (lane 7) or in Hanks’ solution (lane 8), and the latter was followed by re-incubation in 10% FCS/DMEM for 120 min (lane 9). After incubation, the cells were lysed and analysed by immunoblotting using antibody against LC3. A representative experiment repeated twice with duplicated dishes is shown. (B) HeLa cells (open squares with solid lines) and ES cells (open circles with dashed lines) were incubated in Hanks’ solution for the indicated time and a portion of the cells was subjected to immunoblotting using antibody against LC3. The amount of LC3-II was quantified by densitometry (left vertical axis). The remaining HeLa cells were fixed with 2.5% glutaraldehyde for conventional electron microscopy. The area of sections of autophagosomes/autolysosomes was measured on the electron micrographs and is indicated as bars (right vertical axis). (C) HeLa cells transiently transfected with Myc-LC3 were cultured in 10% FCS/DMEM (a) or in Hanks’ solution (b) at 37°C for 90 min. The cells were fixed and permeabilized for immunofluorescence confocal microscopy using anti-Myc epitope antibody and rhodamine-conjugated secondary antibody. Bar, 20 µm.
