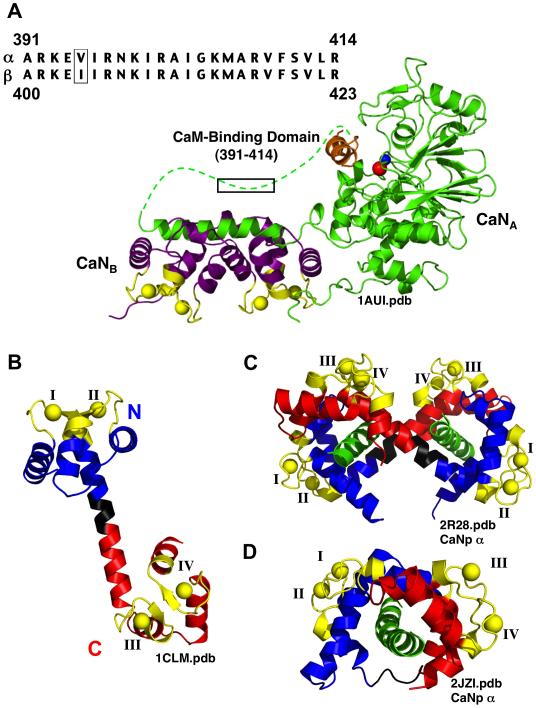
FIGURE 1.
Structures of Calmodulin and Calcineurin. (A) Ribbon diagram of heterodimeric Calcineurin (1AUI.pdb), showing CaNA (green) and CaNB (purple). The dotted green line represents CaN residues that are absent from the electron density. The calcium-binding sites of CaNB are shown in yellow. Two metals ions, zinc (red) and iron (blue), are located in the active site of CaN; in the absence of CaM, this site is held inactive by the auto-inhibitory domain (orange). The sequence of the CaM-binding domains of α–CaN (residues 391-414) and β–CaN (residues 400-423) is boxed. (B) Ribbon diagram of (Ca2+)4-CaM (Paramecium CaM, 1CLM.pdb, 1.8 Å). Calcium-binding sites (yellow) I and II are in the N-domain (blue), and sites III and IV are in the C-domain (red). The N and C domains are linked via a flexible linker (black). (C) Ribbon diagram of (Ca2+)4-CaM bound to the CaM-binding domain of α–CaN (αCaNp, green) solved using X-ray crystallography (2R28.pdb). αCaNp1 is contacted by the N-domain of one CaM molecule (N1) and the C-domain of a second CaM molecule (C2), and αCaNp2 is contacted by N2 and C1. (D) Ribbon diagram of (Ca2+)4-CaM bound to αCaNp peptide (green) solved using NMR (2JZI.pdb). Ribbon diagrams were created using MacPymol™ (DeLano Scientific).