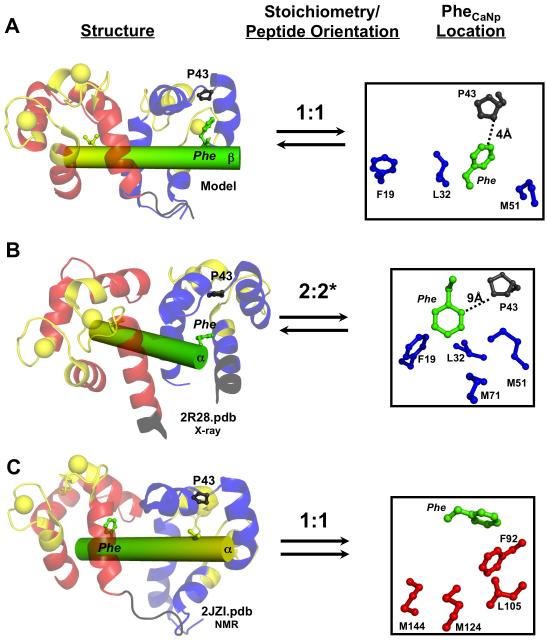
FIGURE 7.
Comparison of the CaM-βCaNp binding interface in three complexes. Ribbon diagram of CaM (N-domain; blue, C-domain; red) bound to βCaNp (green/yellow), stoichiometry and peptide orientation, and location of the sole Phe residue (βCaNpPhe) for the model, (A, βCaNp), the crystal structure (B, αCaNp, 2R28.pdb, *indicates only one CaM-αCaNp unit shown in Figure 1C) and the NMR solution structure (C, αCaNp, 2JZI.pdb). In each structure, I/V at peptide position 5 (yellow) and F at position 20 (green) of βCaNp, P43 (gray), and FLMMN residues (blue; [red for FLMMC residues in C]) are shown. Anti-parallel binding is represented with opposite facing arrows; parallel binding is represented with arrows facing same direction.