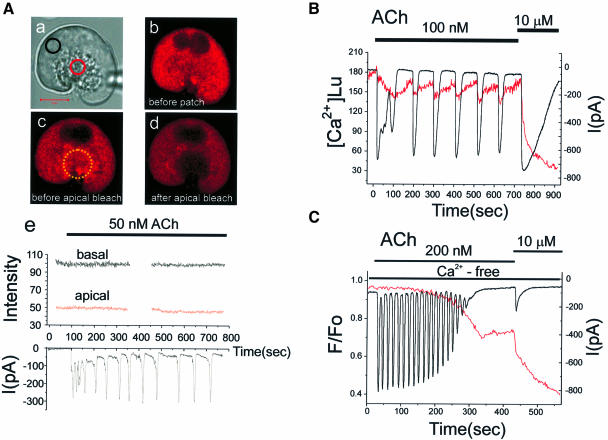
Fig. 7. Repetitive short-lasting cytosolic Ca2+ spikes evoked by a low ACh concentration are associated with no or very small changes in the Ca2+ concentration inside the ER. (A) (a) Transmitted light picture of the cell investigated, with the two regions of interest identified by the coloured circles. The fluorescence intensity images before (b) and after (c) the establishment of the patch–clamp whole-cell recording configuration as well as after the apical bleaching (d) are shown. (e) The Ca2+-sensitive fluorescence intensity from Mag-fluo 4 inside the ER before and during stimulation with 50 nM ACh in both the basal and apical regions (upper part) as well as the Ca2+-dependent whole-cell current (lower part). (B) 100 nM ACh generated more substantial Ca2+-dependent current oscillations close to the maximal activation of the current with 10 µM ACh. In this particular cell, we observed small decreases in the Ca2+ concentration of the ER, recorded using the ratiometric dye Mag-fura 2 (351 and 364 nm excitation) and calibrated as described previously (Park et al., 1999). (C) Measurement of the ER Ca2+ concentration (Mag-fluo 4) and the Ca2+-dependent current oscillations in a cell exposed to an external Ca2+-free solution. In Ca2+-free solutions, low doses of ACh (25–100 nM) failed to evoke Ca2+ oscillations and 200 nM ACh had to be used to generate repetitive cytosolic Ca2+ spikes. Individual short-lasting Ca2+-dependent current spikes were not associated with any measurable drop in the Ca2+ concentration in the ER store, but after ∼2 min of repetitive spiking the ER Ca2+ concentration began to decrease. As the ER was losing Ca2+, the spikes became smaller and finally disappeared. After the Ca2+ oscillations had ceased, there was a further decrease in the ER Ca2+ concentration during a period of non-oscillatory elevation in the cytosolic Ca2+ concentration. Finally, a supramaximal ACh (10 µM) application caused a marked further Ca2+ release from the ER.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.