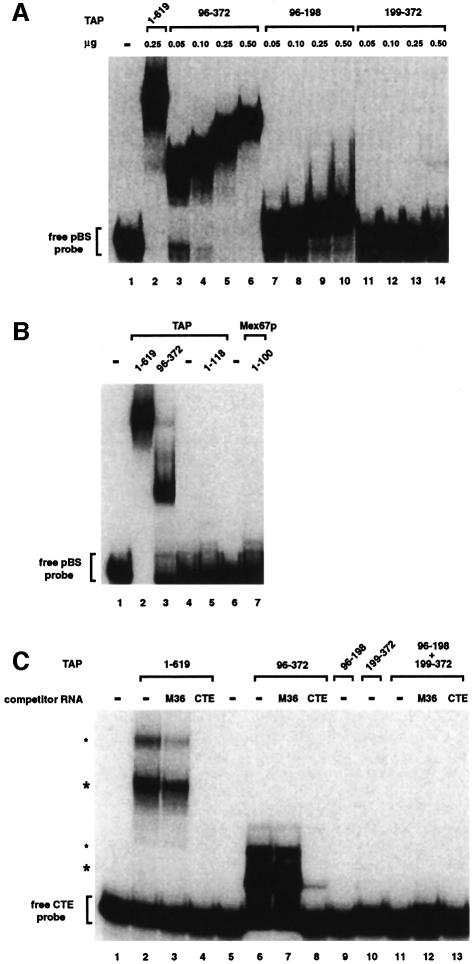
Fig. 4. RNA-binding properties of TAP fragments. (A) The RNP domain exhibits general RNA binding affinity, while the LRR domain does not. An electrophoretic mobility retardation assay was performed with a labeled RNA probe derived from pBluescribe and the purified recombinant proteins indicated above the lanes with the quantities used. Binding reactions were performed in the presence of competitor tRNA (5 ng/µl) and herring sperm single-stranded DNA (0.5 ng/µl, mean size 600 nucleotides). The position of the free RNA probe (lane 1) is shown on the left. (B) A gel-mobility retardation assay was performed as described in (A). The purified recombinant proteins are indicated above the lanes and symbols are as in (A). (C) The RNP domain requires the LRR domain in cis for specific binding to the CTE RNA. A gel-mobility assay was performed with a labeled CTE RNA probe and the purified recombinant proteins are indicated above the lanes. For each protein, binding reactions were performed in the absence and presence of either CTE or M36 competitor RNAs (200 ng/µl) as indicated. All binding reactions contain competitor tRNA (200 ng/µl) and herring sperm single-stranded DNA (20 ng/µl). The concentration of the recombinant proteins in the binding reactions was 5 ng/µl, with the exception of fragment 96–372, which was tested at 2.5 ng/µl in order to have equimolar amounts relative to TAP. The positions of the free CTE RNA probe (lane 1) and of the TAP–CTE RNA complexes (asterisks) are indicated on the left. The upper complexes (small asterisks) may represent two molecules of TAP bound to the CTE RNA. Note that full-length TAP (1–619), TAP fragments 1–118 and Mex67p fragment 1–100 were fused to GST. Fragment 199–372 was expressed with a C-terminal His6 tag. Fragments 96–372 had no tag. Fragment 96–198 had no tag in (A), but was fused to GST in (C).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.