Abstract
BACKGROUND AND PURPOSE
There is growing interest in the physiological functions of flavonoids, especially in their effects on cognitive function and on neurodegenerative diseases. The aim of the current investigation was to evaluate the role of the flavonoid baicalein in long-term potentiation (LTP) in the hippocampal CA1 region and cognitive behavioural performance.
EXPERIMENTAL APPROACH
Effects of baicalein on LTP in rat hippocampal slices were investigated by electrophysiological methods. Phosphorylation of Akt (at Ser473), the extracellular signal-regulated kinase (ERK1/2) and the transcription factor cAMP response element-binding protein (CREB) (at Ser133) were analysed by Western blot. Fear conditioning was used to determine whether baicalein could improve learning and memory in rats.
KEY RESULTS
Baicalein enhanced the N-methyl-d-aspartate glutamate receptor-dependent LTP in a bell-shaped concentration-dependent manner. Addition of the lipoxygenase metabolites 12(S)-HETE and 12(S)-HPETE did not reverse these effects of baicalein. Baicalein treatment enhanced phosphorylation of Akt during induction of LTP with the same bell-shaped dose–response curve. LTP potentiation induced by baicalein was blocked by inhibitors of phosphoinositide 3-kinase. CREB phosphorylation was also increased in the CA1 region of baicalein-treated slices. Baicalein-treated rats performed significantly better than controls in a hippocampus-dependent contextual fear conditioning task. Furthermore, baicalein treatment selectively increased the phosphorylation of Akt and CREB in the CA1 region of hippocampus, but not in the prefrontal cortex, after fear conditioning training.
CONCLUSIONS AND IMPLICATIONS
Our results demonstrate that the flavonoid baicalein can facilitate memory, and therefore it might be useful in the treatment of patients with memory disorders.
Keywords: baicalein, long-term potentiation, 12-lipoxygenase, phosphoinositide 3-kinase, hippocampus, fear conditioning, NMDA receptor
Introduction
A growing body of evidence suggests that memory and cognitive impairment is associated with both physiological aging and pathological conditions in the central nervous system, including as brain ischaemia, Alzheimer's disease and Parkinson's disease. Therefore, there is considerable interest in the development of new drugs to improve cognitive performance in impaired individuals (Marshall, 2004). Recently, interest has focused on a group of phytochemicals found in normal diets, known as flavonoids, capable of inducing improvements in memory acquisition, consolidation, storage and retrieval (Spencer, 2009). Previous studies have shown that the extracts of flavonoid-rich plant or specific flavonoid molecules, such as grape (Joseph et al., 2009), green tea (Li et al., 2009), pomegranates (Hartman et al., 2006), fisetin (Maher et al., 2006), epicatechin (van Praag et al., 2007), oroxylin A (Kim et al., 2008) are able to improve memory and synaptic plasticity through their interactions with neuronal signalling pathways pivotal in controlling long-term potentiation (LTP) and memory.
Long-term potentiation is a manifestation of activity-dependent synaptic plasticity and has increasingly been a prime target for studies on learning and memory in the hippocampus and other brain regions of rodents (Bliss and Collingridge, 1993; Malenka and Bear, 2004). LTP is a long-lasting increase in synaptic strength that can be induced by tetanic stimulation of afferent fibres. Previous studies have shown that LTP triggered by high-frequency stimulation (HFS) or theta-burst stimulation (TBS) in hippocampal CA1 area requires postsynaptic molecular mechanisms, such as activation of N-methyl-d-aspartate (NMDA) receptors and involvement of the phosphoinositide 3-kinase (PI3K)/Akt signalling cascade. It has been proposed that Ca2+ influx through NMDA receptors triggers a series of intracellular signalling cascades, including the PI3K/Akt pathway, which lead to increased synaptic strength, which is believed to play a pivotal role in NMDA receptor-dependent LTP in the hippocampal CA1 region (Man et al., 2003). Recent reports have revealed that flavonoids and other small molecules or drugs affect LTP, and consequently memory and cognitive performance, through their interactions with these signalling pathways (Maher et al., 2006; Mans et al., 2010).
Scutellaria baicalensis Georgi (Huangqin) is a herb with antibacterial and anti-inflammatory properties, widely used for many centuries in China and Japan. Baicalein is the most effective antioxidant among the major flavonoids isolated from the roots of S. baicalensis. Herbal preparations of baicalein have been used to improve deficiencies of learning and memory for thousands of years in traditional Chinese medicine. In our previous studies, baicalein alleviated cognitive deficits induced by chronic cerebral hypoperfusion and protected neurons against ischaemic injury by activating the PI3K/Akt pathway in rats (Liu et al., 2007; 2010;). Additionally, a microarray analysis of gene expression revealed that the expression of certain genes related to learning and memory were normalized, in ischaemic mice brain, after treatment with baicalein (Wang et al., 2004b). A recent study also showed that one dose pretreatment of baicalein attenuated amnesia, induced by β-amyloid peptide-(25–35) (Wang et al., 2004a). However, the effect and mechanism of baicalein on learning and memory in normal animals remain unclear. In the present study, we have investigated the effect of baicalein on LTP in the CA1 region of rat hippocampal slices and cognitive behavioural performance in adult rats, as well as the underlying molecular mechanisms.
Methods
Electrophysiological recordings
All animal care and experimental protocols were in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and approved by the Review Committee for the Use of Human or Animal Subjects of Huazhong University of Science and Technology. Hippocampal slices were prepared from Sprague-Dawley rats (6–8 weeks old) as previously described (Liu et al., 2009) with some modification. Briefly, brains were rapidly removed and coronal brain slices (400 µm thickness) containing hippocampus were cut using a vibrating blade microtome in ice-cold artificial cerebrospinal fluid (ACSF) containing (mM) 119 NaCl, 3.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3 and 11 glucose that was bubbled continuously with 95%O2–5%CO2 to adjust pH to 7.4. After 1.5 h of recovery at 27°C, an individual slice was transferred to a submerged recording chamber and continuously superfused with oxygenated ACSF at 30°C at a rate of 3–4 mL·min−1. Field excitatory postsynaptic potentials (fEPSPs) were evoked by a constant stimulation in the Schaffer collaterals with a bipolar electrode and recorded in the stratum radiatum layer of CA1 with a glass micropipette filled with 3 M NaCl. Stimulation intensities were chosen to produce a fEPSP with a slope that was 30–35% of that obtained with maximal stimulation.
Long-term potentiation was induced electrically by one of the following two protocols: (i) The HFS protocol used to induce LTP consisted of three 1 s, 100 Hz stimulus trains separated by a 30 s interval between trains. This protocol has been used previously to induce NMDA receptor-dependent LTP (Malenka and Bear, 2004; Berberich et al., 2007); (ii) The TBS protocol used to induce LTP contained two trains with 10 s intervals between trains. Each train consisted of 10 bursts separated by 200 ms (theta rhythm). Each burst included five 100 Hz pulses delivered at 30–35% of maximal stimulus intensity. A similar protocol has been used to induce NMDA receptor-dependent LTP in an earlier study (Morgan and Teyler, 2001).
Paired stimuli (25, 50, 75, 100 ms interval) were delivered to the Schaffer collateral and the paired-pulse ratio (PPR) was calculated as the ratio between the mean slope of the second fEPSP (fEPSP2) over the first fEPSP (fEPSP1). The initial slope of the fEPSP was measured and expressed as a percentage change from the baseline level, calculated from an average of the last 20 min of the baseline recording period. The degree of LTP for each experiment was measured at 60 min after the tetanic stimulation.
Western blotting
After the electrophysiological studies, CA1 region of hippocampal slices was removed for Western blotting. After behavioural studies, the CA1 region of hippocampus and prefrontal cortex were removed for Western blotting, 15 min after contextual fear conditioning training. The procedures were processed according to our previous protocols with some modifications (Cai et al., 2008; Liu et al., 2009). Briefly, hippocampus and cortex were homogenized with buffer containing: 50 mM Tris-base (pH 7.4), 100 mM NaCl, 1% NP-40, 10 mM EDTA, 20 mM NaF, 1 mM PMSF, 3 mM Na3VO4 and protease inhibitors. Total protein was estimated by Coomassie blue protein-binding assay (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). Then, the samples were mixed with sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and stored at −80°C until electrophoresis. Samples (20 µg) were analysed by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Schleicher and Schuell, Keene, NH, USA). After blocking with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature, transferred membranes were incubated overnight at 4°C with appropriate primary antibodies against β-actin (1:3000) (Upstate Biotechnology, Lake Placid, NY, USA), anti-phospho-Akt (Ser473) (1:500), anti-Akt (1:1000), anti-cAMP response element-binding protein (CREB; 1:400); anti-phospho-CREB (Ser133) (1:400) (Cell Signaling, San Francisco, CA, USA), anti-extracellular signal-regulated kinase (ERK1/2; 1:500) (Abcam, Cambridge, UK) and anti-phospho-ERK1/2 (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following three washes with TBST, membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) in TBST with 1% non-fat milk for 1 h at room temperature and reacted with enhanced chemiluminescence reagents (Super Signal West Pico; Pierce Chemical Co., Rockford, IL, USA) and visualized with X-ray films (Kodak X-Omat, Rochester, NY, USA). The films were scanned and the optical densities of detected bands were quantified using NIH Image J software (National Institutes of Health, Bethesda, MD, USA). The results were normalized to the quantity of β-actin in each sample lane. All assays were performed at least three times.
Fear conditioning task
Sprague-Dawley rats (6–8 weeks old) were used for these studies. Animals were housed individually in environmentally controlled conditions (22°C, 12 h light–dark cycle with light cycle from 0700 to 1900 h and dark cycle from 1900 to 0700 h with ad libitum access to standard laboratory chow and water. Rats were allowed 1 week to adapt to their new environment before experiments began. The fear conditioning procedures were processed according to previous protocols with some modifications (Barha et al., 2010).
Fear conditioning took place in an observation chamber (32 × 26 × 22 cm), consisting of aluminium (side walls) and Plexiglass (hinged front door, and rear wall). The chamber was enclosed within a sound-attenuating box located in a quiet room. A video camera was positioned above the chamber to record the behaviour of the animal for video scoring. The floor of the chamber consisted of 16 stainless steel rods spaced 1.6 mm apart (centre-to-centre). Rods were wired to a shock source and solid-state grid scrambler for the delivery of foot shock. Each chamber was illuminated by a single house light positioned in the top centre of one wall. In the left corner of the same wall, a speaker connected to a programmable audio generator was located. The background noise was supplied by ventilation fans in the box. Rats were handled in the room where conditioning took place for 5 min per day for 3 days. The habituation procedure was introduced to completely familiarize the rats with the stimuli of the experimental room, and thus prevent any interference of uncontrolled novel stimuli during the experiments.
This experiment was conducted over 2 days, conditioning day and testing day. On day 1 (the conditioning day), rats were injected with baicalein or vehicle. Twenty minutes later, they were placed into the chamber and the house light was turned on. After a 3 min acclimatizing period, they were given two presentations of the tone conditioned stimulus (80 dB, 30 s) co-terminating with a foot shock with 60 s intervals. Each shock was 0.75 mA and 2 s duration. Rats were left in the conditioning chamber for 30 s after termination of the procedure and then returned to their home cage. To assess contextual fear conditioning, 24 h after conditioning, rats were placed into the conditioning chamber and observed for 3 min. One hour later, the animals were assessed for cued fear conditioning in a novel test chamber, with different contextual cues, during a 3 min presentation of the conditioned stimulus. Memory was assessed by measurement of time spent freezing. Freezing was defined as the complete absence of activity except for respiratory movement. The data were converted to the percentage of samples scored as freezing.
Rats were randomly assigned to one of five treatment groups (n= 8 per group) and received a single i.p. injection of either vehicle or different doses (10, 20, 50 and 100 mg·kg−1) of baicalein. Baicalein that dissolved in dimethyl sulfoxide (DMSO) or a corresponding volume of vehicle (DMSO) was administered 20 min before training. These doses and dosing time were chosen based on the pharmacokinetic profile of baicalein in the rat defined in an earlier study (Tsai et al., 2002).
Data analysis
Data are presented as means ± SEM. One-way anova or paired t-test was used for the statistical analysis by employing SPSS 10.0 software. Differences at the P < 0.05 level were considered statistically significant.
Materials
Baicalein (MW: 270.24, purity above 98%) was obtained from Sigma (St. Louis, MO, USA). 12(S)-hydroperoxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid [12(S)-HPETE] and 12(S)-hydroxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid [12(S)-HETE] were obtained from Cayman Chemicals (Ann Arbor, MI, USA). d-2-amino-5-phosphonovaleric acid (D-APV) and wortmannin were purchased from Sigma (St. Louis, MO, USA). LY294002 was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). MK-801 was kindly provided by NIMH synthesis program. Other general agents were purchased from commercial suppliers. Baicalein was dissolved in dimethyl sulfoxide (DMSO; Sigma), and the final concentration of DMSO in all groups was no more than 0.1%. The same volume of DMSO was used as control.
Results
Baicalein enhances HFS-induced LTP in rat hippocampal CA1 region
In the first set of experiments, the effect of baicalein on basal excitatory synaptic transmission in the CA1 region of hippocampal slices was examined. After establishing a stable baseline for 20 min, the fEPSP was recorded for 20 min under perfusion with ACSF containing various concentrations of baicalein (0.1, 1, 10, 50 µM). No significant changes in the fEPSP slope were observed after baicalein perfusion (Figure 1A and B). These results suggest that baicalein did not affect basal synaptic transmission.
Figure 1.
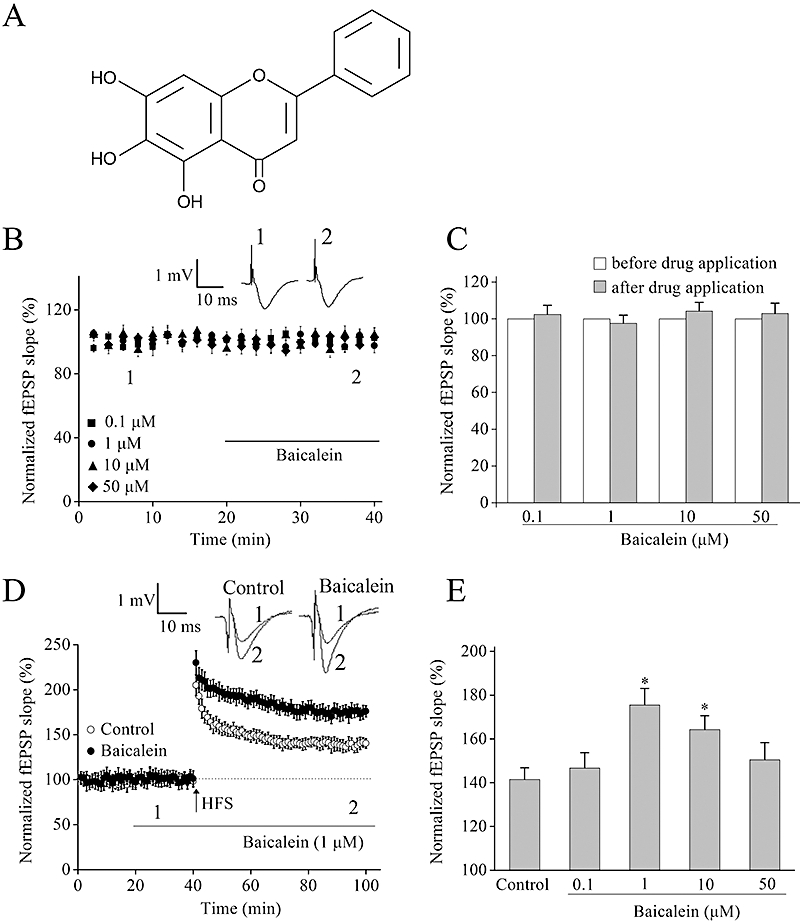
Pretreatment with baicalein enhances long-term potentiation (LTP) in the hippocampal CA1 region. (A) Chemical structure of baicalein. (B) Baicalein (0.1, 1, 10 and 50 µM) had no effect on basal synaptic transmission. After establishing a stable field excitatory postsynaptic potential (fEPSP) baseline for 20 min, baicalein was perfused continuously (indicated by the bar) to individual slices. Insets, the sample traces before (1) or after (2) perfusion with baicalein. (C) Summary of averaged fEPSP slope from hippocampal slices incubated with different concentrations of baicalein (0.1, 1, 10 and 50 µM). No significant change was shown after drug application in each group, n= 6 for each group. The mean fEPSP slope before drug application (1 to 20 min) was normalized as 100% and the fEPSP slope at every time point was normalized to it. (D) Effect of baicalein (1 µM) on LTP in CA1 region of rat hippocampus. The superimposed fEPSPs in the upper portion show typical recordings from experiments taken at the time indicated by the number. (E) Summary data of the level of LTP 60 min after high-frequency stimulation (HFS) in the absence or presence of various concentrations of baicalein. *P < 0.05 versus control. Each point was the normalized mean ± SEM of 6–8 slices.
To evaluate whether baicalein could affect synaptic plasticity in normal animals, we next examined the effect of baicalein on HFS-induced LTP in hippocampal CA1 region of rats. As shown in Figure 1C and D, pre-incubation of hippocampal slices with baicalein for 20 min enhanced the HFS-induced LTP in a bell-shaped, concentration-dependent manner with the effect reaching a maximum at 1 µM and persisting for at least 60 min (Figure 1D).
Baicalein does not affect input–output relationship and paired-pulse facilitation (PPF) in the hippocampal CA1 region of rat
To determine whether baicalein can affect the input–output relationship, which reflects the efficacy of synaptic transmission, the fEPSP was recorded under different stimulus intensity. Baicalein (1 µM) did not alter the input–output relationship at any stimulus intensity (n= 6, Figure 2A and B).
Figure 2.
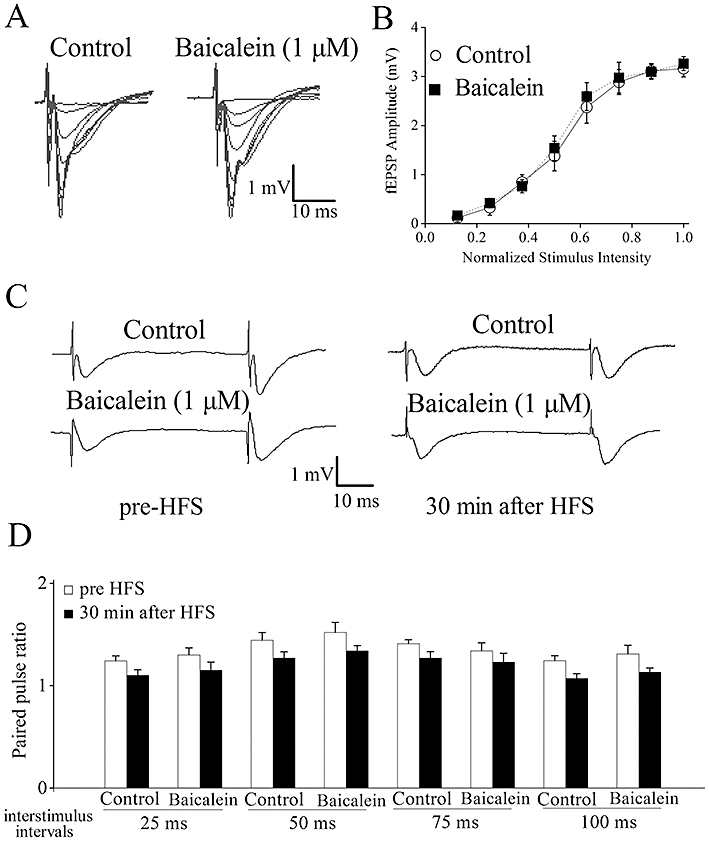
Baicalein treatment does not affect baseline evoked responses or paired-pulse facilitation. (A) Typical superimposed field excitatory postsynaptic potential (fEPSP) recorded from the CA1 region in the presence and absence of 1 µM baicalein by increasing stimulation intensity. (B) Input–output curves illustrating the relationship between the stimulation and evoked response for fEPSPs recorded from control and baicalein-treated slices. No significant differences were observed. (C) Typical fEPSPs are shown from experiments at 50 ms interpulse interval before and after high-frequency stimulation (HFS) stimulation. (D) Paired-pulse facilitation (PPF) was measured by varying the intervals between pairs of stimuli before and after HFS stimulation. No significant differences were observed. Each point was the normalized mean ± SEM of five slices.
Long-term potentiation reflects a persistent enhancement in synaptic strength in which both presynaptic and postsynaptic mechanisms might be involved (Malenka and Nicoll, 1999). PPF is a sensitive measure of altered neurotransmitter release probability, a form of short-term presynaptic plasticity and we used this protocol to examine whether presynaptic mechanisms were involved in LTP facilitation induced by baicalein. The PPR in slices exposed to DMSO or baicalein at baseline and 30 min after HFS stimulation was examined. In control slices (DMSO), PPR was significantly decreased after HFS stimulation, indicating an enhanced neurotransmitter release within LTP (Figure 2C and D). In slices pretreated with 1 µM baicalein for 20 min, PPR decreased similarly after HFS stimulation (n= 5, P > 0.05 vs. control, Figure 2C and D). There was no difference in the effect of LTP on PPR between control and baicalein-treated slices, indicating that the effects of baicalein on LTP were unlikely to result from presynaptic changes in probability of transmitter release.
NMDA receptors are involved in baicalein-facilitated LTP
At CA3-CA1 synapses, LTP induced by 100 Hz tetanic stimulation depends primarily on Ca2+ influx through NMDA receptors (Collingridge et al., 1983) and this potentiation is prevented by the blockade of postsynaptic NMDA receptors. Consistent with previous observations, when NMDA receptor antagonists D-APV (50 µM) and MK-801 (10 µM) were applied, 100 Hz tetanic stimulation could not induce LTP (D-APV: 97.4 ± 8.2%, n= 6; MK-801: 98.3 ± 7.4%, n= 6; P < 0.05 vs. control; Figure 3A and B). Pre-incubation with D-APV (50 µM) or MK-801 (10 µM) for 10 min before baicalein (1 µM) application completely prevented baicalein-facilitated LTP (D-APV + baicalein: 101.2 ± 8.5%, n= 6; MK-801 + baicalein: 103.1 ± 8.4%, n= 6; P < 0.05 vs. baicalein alone; Figure 3A and B).
Figure 3.
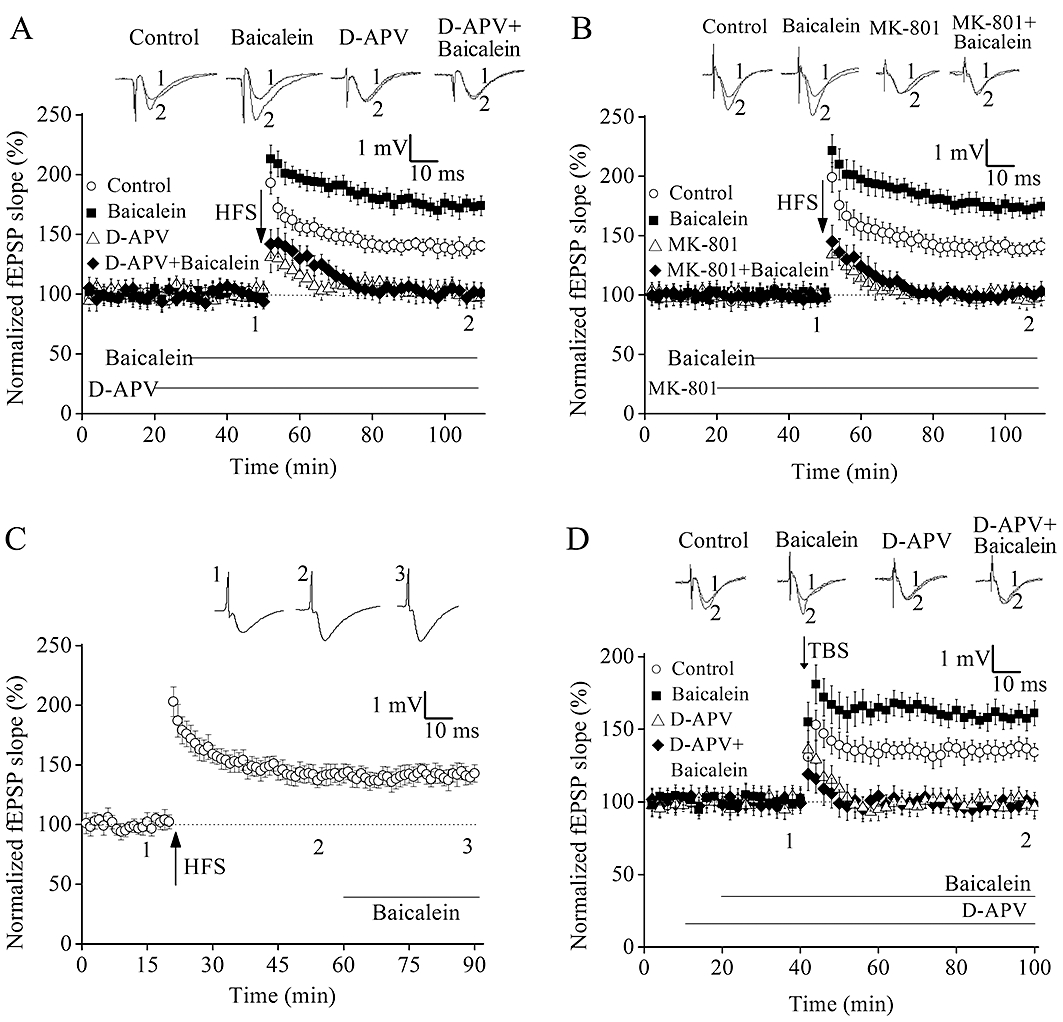
N-methyl-d-aspartate (NMDA) receptors contribute to baicalein-facilitated long-term potentiation (LTP). (A) Changes in slopes of field excitatory postsynaptic potential (fEPSP) following high-frequency stimulation (HFS) in the presence and absence of 1 µM baicalein and 50 µM d-2-amino-5-phosphonovaleric acid (D-APV). (B) Changes in slopes of fEPSP following HFS in the presence and absence of 1 µM baicalein and 10 µM MK-801. (C) Average effects of 1 µM baicalein applied for 30 min after the induction of LTP. (D) Changes in slopes of fEPSP following theta-burst stimulation (TBS) in the presence and absence of 1 µM baicalein and 50 µM D-APV. The superimposed fEPSP in the upper portion are recordings from representative experiments taken at the time indicated by the number. Each point was the normalized mean ± SEM of six slices.
To determine whether the baicalein-facilitated LTP was time-dependent, application of baicalein (1 µM) application was delayed until 40 min after HFS. On average, the slope of fEPSP measured 40 min after HFS was 143 ± 8.5% of pre-stimulation baseline, which was not significantly different from that of LTP recorded in slices after application of 1 µM baicalein for 30 min (139 ± 6.4% of pre-stimulation baseline; n= 6, Figure 3C). These results demonstrate that baicalein barely affected synaptic response if applied after LTP has been established, and baicalein is needed during the period of HFS stimulation in order to facilitate LTP.
In order to confirm the role of baicalein, hippocampal LTP was induced by the other stimulation pattern, TBS, which is a more physiologically relevant stimulus (Raymond, 2007). Several studies have reported that two trains of TBS results in LTP that is completely blocked by NMDA receptor antagonists (Morgan and Teyler, 2001). As expected, it was found that incubation of baicalein (1 µM) alone for 20 min exhibited a dramatic increase in the magnitude of TBS-LTP (control: 134.1 ± 6.1%; baicalein: 161.1 ± 8.6%; n= 6 for each group, P < 0.05, Figure 3D). Furthermore, pre-incubation of D-APV (50 µM) for 10 min before baicalein application robustly blocked baicalein-facilitated LTP (D-APV + baicalein: 96.4 ± 8.1%; n= 6, P < 0.05 vs. baicalein alone, Figure 3D).
12-Lipoxygenase inhibition is not required for baicalein-induced LTP enhancement
Baicalein is known as a 12-lipoxygenase (12-LO) inhibitor and widely used to decrease the generation of 12(S)-hydroperoxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid [12(S)-HPETE] and 12(S)-hydroxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid [12(S)-HETE] (two metabolites of 12-LO) in cell proliferation studies (Dailey and Imming, 1999). We therefore investigated whether these metabolites contributed to the effect of baicalein. Pretreatment of hippocampal slices with 250 nM 12(S)-HETE (Figure 4A and B) or 250 nM 12(S)-HPETE (Figure 4C and D) for 10 min did not affect the amplitude of LTP measured 60 min after HFS, with or without 1 µM baicalein. A higher (1 µM) or lower concentration (100 nM) of 12(S)-HETE or 12(S)-HPETE did not reverse the enhancement of LTP (data not shown).
Figure 4.
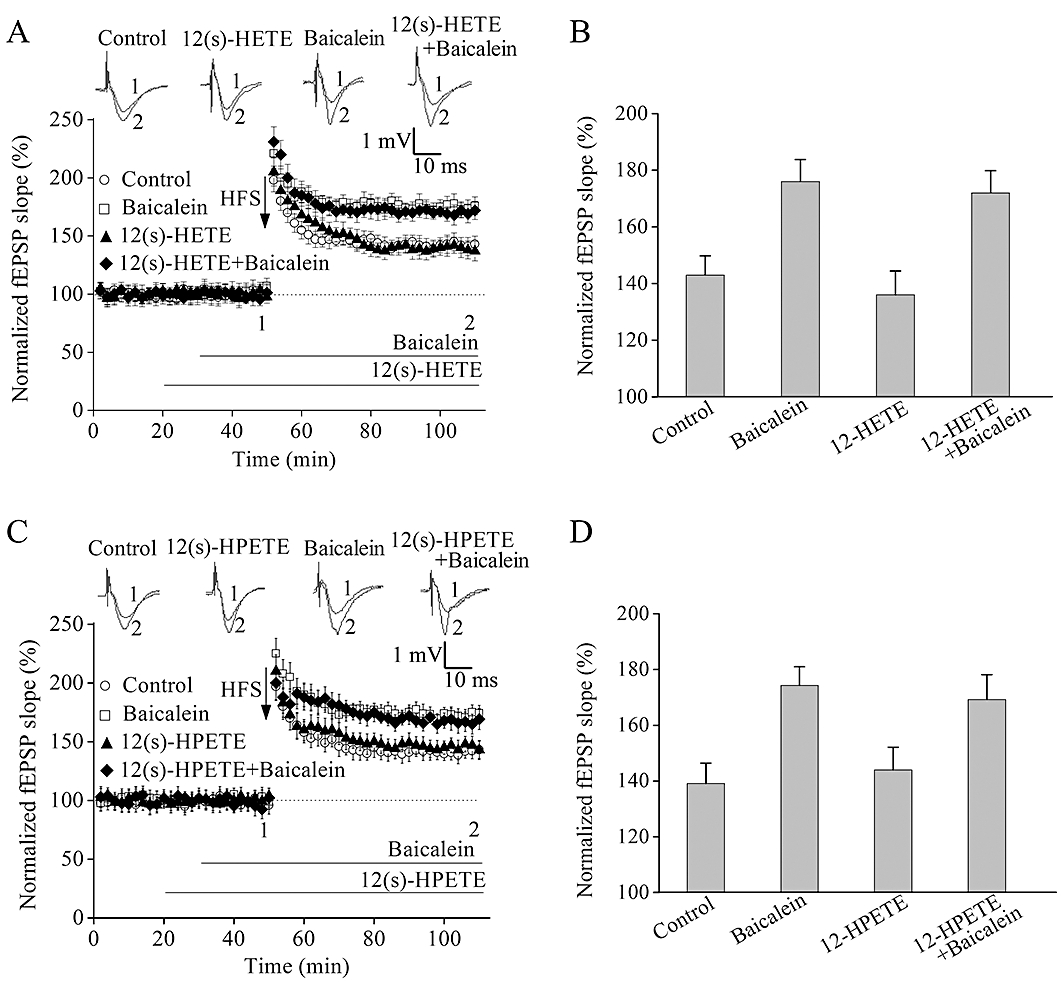
Addition of 12(S)-HETE or 12(S)-HPETE did not affect baicalein-induced long-term potentiation (LTP) enhancement. (A) Pre-incubation of slices with 12(S)-HETE (250 nM) did not affect the induction of high-frequency stimulation (HFS)-induced LTP and baicalein-facilitated LTP. (B) Summary data of the average field excitatory postsynaptic potential (fEPSP) slope after HFS relative to untreated, baicalein, 12(S)-HETE, the combination of 12(S)-HETE and baicalein. (C) 12(S)-HPETE did not block HFS-induced LTP and baicalein-facilitated LTP. (D) Summary data of the average fEPSP slope after HFS relative to untreated, baicalein, 12(S)-HPETE, the combination of 12(S)-HPETE and baicalein. The superimposed fEPSP in the top portion showed respective recordings from representative experiments taken at the time indicated by the number. Each point was the normalized mean ± SEM of six slices.
Activation of the PI3K pathway is required for baicalein-induced LTP enhancement
A number of recent studies have shown that PI3K is involved in synaptic plasticity, and some flavonoids such as baicalein and the citrus flavanone hesperetin activate the PI3K pathway in cortical and hippocampal neurons (Vauzour et al., 2007; Spencer, 2009; Liu et al., 2010). In our next experiments, the effects of baicalein on levels of phosphorylation of Akt (Ser473) and total Akt were measured by Western blotting analyses. HFS stimulation induced a transient phosphorylation of Akt at Ser473, which reached the maximum at 5 min after LTP and returned to baseline values within 60 min (Figure 5A and B). Akt phosphorylation was further increased by baicalein (1 µM) pre-incubation after HFS in a time-dependent manner, without any significant change in total Akt expression (Figure 5A and B).
Figure 5.
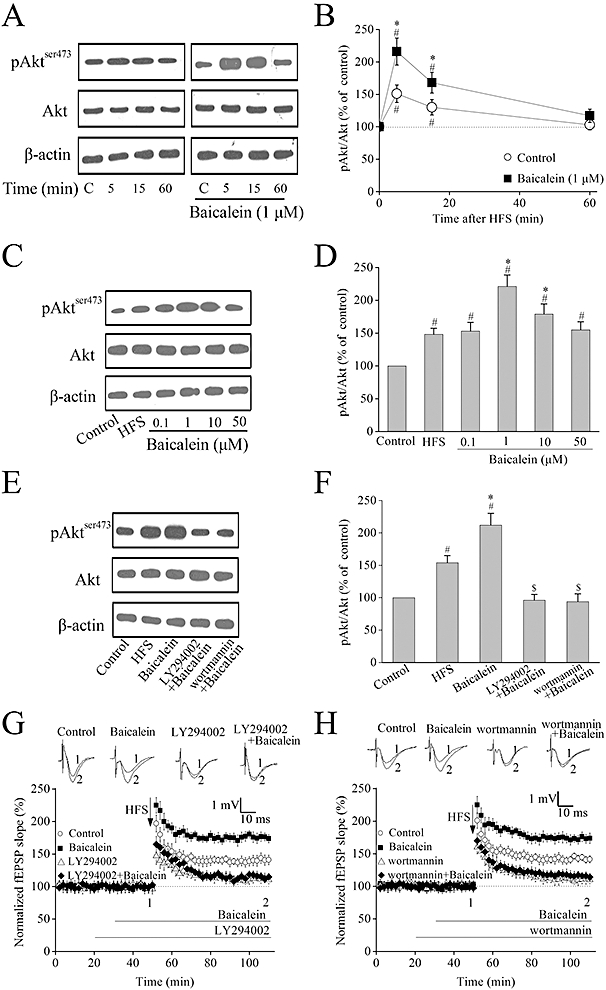
Activation of PI3K pathway is required for baicalein-induced long-term potentiation (LTP) enhancement. (A) Representative images of immunoblots using antibodies against phosphorylated Akt (ser473), total Akt and β-actin. High-frequency stimulation (HFS) stimulation induced a transient activation of Akt phosphorylation. Pre-incubation of baicalein (1 µM) further increased Akt phosphorylation time-dependently. (B) Summary data of phosphorylated Akt and total Akt by densitometry. Data were expressed as percentage of value of controls without stimulation; n= 4. #P < 0.05 versus control (without HFS stimulation), *P < 0.05 versus HFS (without baicalein treatment). (C) Representative images of immunoblots using antibodies against phosphorylated Akt (Ser473), total Akt and β-actin. Baicalein further increased Akt phosphorylation 5 min after HFS in a bell-shaped dose–response manner that peaked at 1 µM. (D) Summary data of phosphorylated Akt and total Akt by densitometry. Data were expressed as percentage of value of controls without stimulation; n= 4. #P < 0.05 versus control, *P < 0.05 versus HFS. (E) Representative images of immunoblots using antibodies against phosphorylated Akt (ser473), total Akt and β-actin. LY294002 (10 µM) or wortmannin (500 nM) completely blocked the baicalein-induced enhancement of Akt phosphorylation 5 min after HFS. (F) Summary data of phosphorylated Akt and total Akt by densitometry. Data were expressed as percentage of value of controls without stimulation; n= 3. #P < 0.05 versus control, *P < 0.05 versus HFS, $P < 0.05 versus baicalein. (G–H) The PI3K inhibitors (LY294002 or wortmannin) totally inhibited baicalein-induced enhancement of LTP. Hippocampal slices were pretreated with either LY294002 (10 µM) or wortmannin (500 nM) for 10 min before addition of 1 µM baicalein following HFS. The superimposed field excitatory postsynaptic potentials (fEPSPs) in the upper portion are recordings from representative experiments taken at the time indicated by the number. Each point was the normalized mean ± SEM of six slices.
This potentiation by baicalein of Akt phosphorylation at 5 min after HFS was dose-dependent but with a bell-shaped profile, peaking at 1 µM, without any significant change in total Akt expression (Figure 5C and D).
Moreover, inhibition of PI3K by LY294002 (10 µM) or wortmannin (500 nM) completely blocked the baicalein-induced enhancement of Akt phosphorylation at 5 min after HFS (Figure 5E and F). We next examined the effects of these PI3K inhibitors on baicalein-enhanced LTP. LTP was markedly reduced in hippocampal slices treated with LY294002 (10 µM) or wortmannin (500 nM) for 30 min before HFS (LY294002: 110.3 ± 6.1%; wortmannin: 112.2 ± 6.7%; n= 6, Figure 5G and H). Furthermore, in slices pre-incubated with LY294002 (10 µM) or wortmannin (500 nM), the enhancement of HFS-LTP induced by baicalein was completely blocked (LY294002 + baicalein: 114.8 ± 5.3; wortmannin + baicalein: 115.3 ± 5.5%; n= 6, Figure 5G and H).
Baicalein increases CREB phosphorylation following HFS in rat hippocampal CA1 region
Long-term potentiation triggered by HFS in the hippocampal CA1 area requires postsynaptic molecular mechanisms, such as activation of the ERKs of the mitogen-activated protein kinase family and of the transcription factor, CREB (Impey et al., 1996; Thomas and Huganir, 2004). Activation of the two distinct signalling pathways of ERK and PI3K lead to the activation of CREB (Spencer, 2009). We therefore assayed ERK1/2 and CREB expression by Western blotting, 5 min after LTP induction, with or without baicalein treatment.
High-frequency stimulation induced an activation of ERK1/2 phosphorylation 5 min after HFS (Figure 6A and B) and pre-incubation of hippocampal slices with baicalein (1 µM) did not affect this phosphorylation (Figure 6A and B). CREB phosphorylation (at Ser133) was also significantly increased following HFS induction (Figure 6C and D) and LTP induction in the presence of baicalein (1 µM) further increased CREB phosphorylation, without any significant change in total CREB expression (Figure 6C and D).
Figure 6.
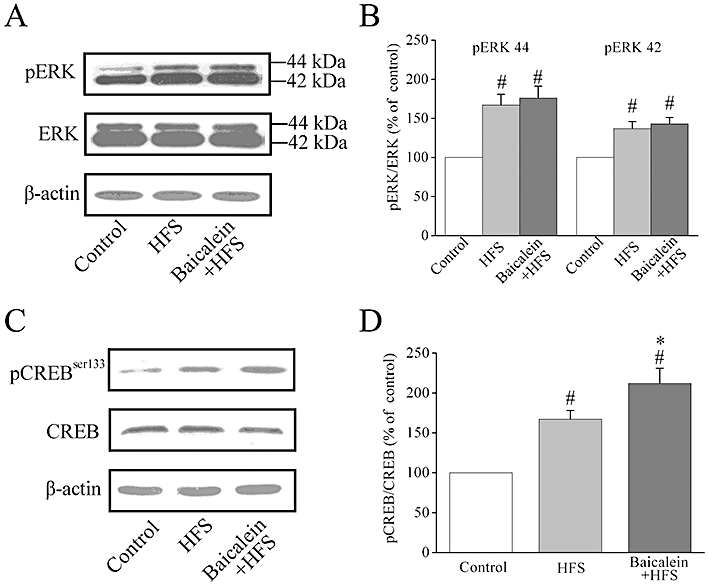
Effects of baicalein treatment on phosphorylation of ERK1/2 and CREB after high-frequency stimulation (HFS) in rat hippocampal CA1 region. (A) Representative images of immunoblots using antibodies against phosphorylated ERK1/2 (pERK1/2), ERK1/2 and β-actin. HFS stimulation induced an activation of ERK1/2 phosphorylation 5 min after HFS. Pre-incubation of hippocampal slices with baicalein (1 µM) did not affect ERK1/2 phosphorylation 5 min after HFS. (B) Summary data of phosphorylated ERK1/2 and total ERK1/2 expression by densitometry. Data were expressed as percentage of value of controls without stimulation. #P < 0.05 versus control, n= 4. (C) Representative images of immunoblots using antibodies against phosphorylated CREB (Ser133), total CREB and β-actin. HFS stimulation induced an activation of CREB phosphorylation 5 min after HFS. Pre-incubation of hippocampal slices with baicalein (1 µM) further increased CREB phosphorylation 5 min after HFS. (D) Summary data of phosphorylated CREB and total CREB expression by densitometry. Data were expressed as percentage of value of controls without stimulation. #P < 0.05 versus control, *P < 0.05 versus HFS, n= 4.
Baicalein improves hippocampus-dependent contextual fear conditioning
To determine whether the electrophysiological and biochemical effects of baicalein seen in hippocampal slices translated into alterations in memory in vivo, we used a hippocampus-dependent contextual fear conditioning task. The animals were trained for fear conditioning 20 min after baicalein treatment. The pre-training administration of baicalein had no effect on freezing behaviour observed during training (data not shown). Twenty-four hours after training, the rats were tested for freezing behaviour. A timeline of the experiment is presented in Figure 7A. Interestingly, baicalein improved contextual fear conditioning with a bell-shaped dose–response profile, with the peak response at the doses of 20 mg·kg−1 (Figure 7B). During the cued fear conditioning test, all groups did not differ in the amount of time spent freezing during the presentation of the tones (Figure 7C).
Figure 7.
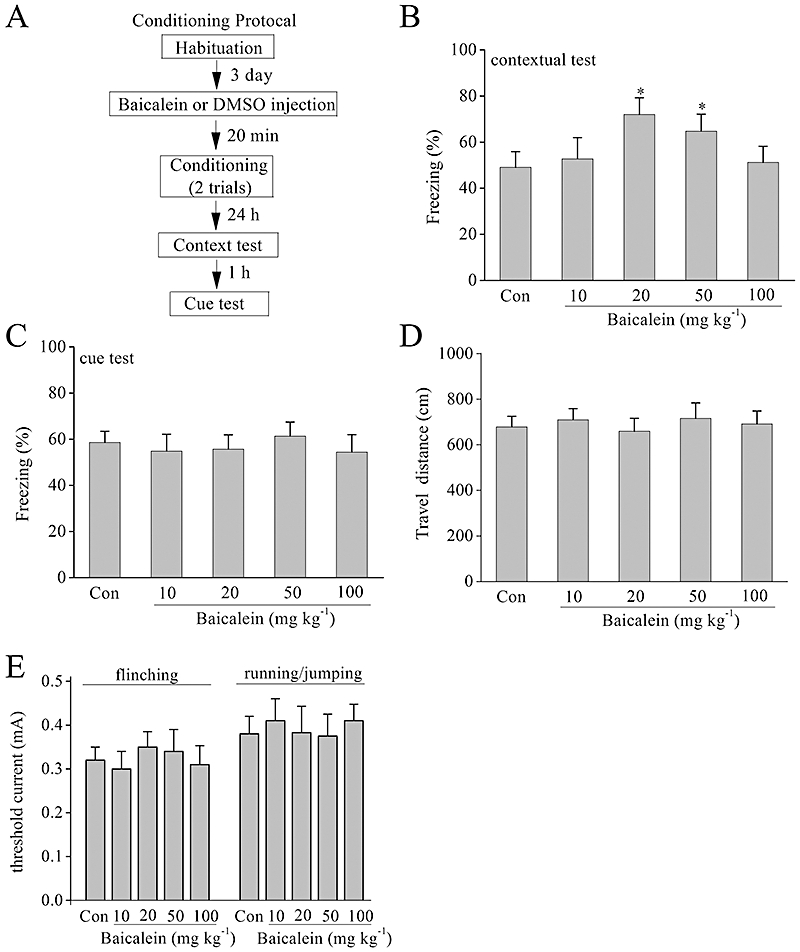
Baicalein improves the learning and memory performances in contextual fear conditioning tests. (A) Experimental design of fear conditioning paradigm. (B) Total percentage of freezing during the 3 min contextual fear conditioning test when tested 24 h after conditioning. Baicalein improved contextual fear conditioning at 20 mg·kg−1 and 50 mg·kg−1. n= 8, *P < 0.05 versus control. (C) Total percentage of freezing during the presentation of the tones in the cued fear conditioning test when tested 1 h after contextual fear conditioning test. Groups did not differ in freezing to the tone during the cued fear conditioning test compared with controls. (D) Distance travelled during the initial 3 min exposure to the training box in an open field test. (E) Pain thresholds. Thresholds of current intensities for flinching and running/jumping are shown.
The enhanced hippocampus-dependent memory formation could be attributable to increased pain sensitivity or motor defects. The rats were exposed to the open field test to analyse their locomotor ability. Distance travelled during the initial 3 min exposure to the training box in an open field test was recorded, and no statistically significant differences were found among the five groups (n= 8, Figure 7D). To determine pain threshold, rats were exposed to electric foots hocks of increasing intensities. The thresholds for running/jumping and flinching in response to the shock did not differ between all groups (n= 8, Figure 7E).
Modulation of Akt and CREB expression in the hippocampus and cortex by baicalein treatment after fear conditioning training
It is well established that hippocampus-dependent memory formation is associated with the activation of the PI3K pathway and increased CRE-mediated gene expression (Impey et al., 1998; Chen et al., 2005). To investigate the mechanisms involved in the modulation of hippocampus-dependent memory by baicalein, Akt and CREB expression were assayed by Western blotting 15 min after fear conditioning training with or without baicalein treatment. In these experiments, rats were divided into three groups: control, training or training with baicalein (20 mg kg−1). Rats in the control group were placed into the conditioning chamber but received no shock.
Fear conditioning training increased CREB and Akt phosphorylation in the CA1 region of hippocampus (Figure 8A and C) but not in prefrontal cortex (Figure 8B and D). Baicalein treatment 20 min before training (20 mg·kg−1) further increased Akt and CREB phosphorylation in the hippocampal CA1 region (Figure 8A and C) but not in the prefrontal cortex (Figure 8B and D).
Figure 8.
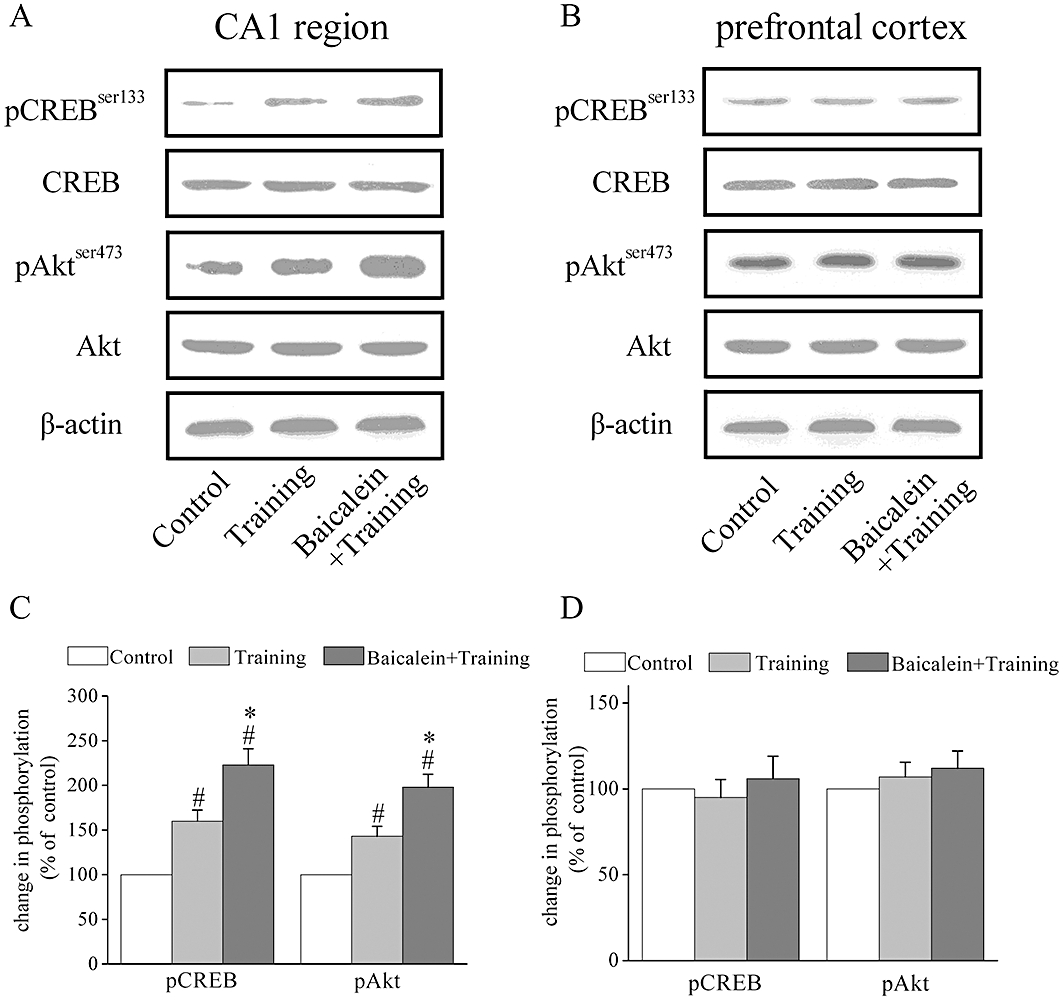
Baicalein treatment results in a selective further increase in the phosphorylation of Akt and CREB in the CA1 region of hippocampus but not in the prefrontal cortex after fear conditioning training. (A–B) Representative images of immunoblots using antibodies against phosphorylated Akt (Ser473), total Akt, phosphorylated CREB (Ser133), total CREB and β-actin. Protein extracts from the hippocampal CA1 region and prefrontal cortex were analysed with Western blotting 15 min after contextual fear conditioning training. Fear conditioning training induced an activation of Akt and CREB phosphorylation in the CA1 region of hippocampus but not in prefrontal cortex. Baicalein treatment 20 min before training (20 mg·kg−1) further increased Akt and CREB phosphorylation in the hippocampal CA1 region but not in prefrontal cortex. (C–D) Summary data of phosphorylated Akt, total Akt, phosphorylated CREB (ser133) and total CREB by densitometry. Data were expressed as percentage of value of controls without training. #P < 0.05 versus control, *P < 0.05 versus training, n= 4.
Discussion
In the present study, we demonstrated for the first time that flavonoid baicalein facilitated the induction of NMDA receptor-dependent LTP in the hippocampal CA1 region in vitro, and that acute administration of baicalein promoted hippocampus-dependent associative memory. The major findings of our study are as follows: (i) application of baicalein facilitated NMDA receptor-dependent LTP in a bell-shaped, concentration-dependent manner without affecting basal synaptic transmission; (ii) the promotion of LTP by baicalein was independent of 12-LO inhibition; (iii) baicalein-facilitated LTP was dependent on the activation of the PI3K pathway; (iv) baicalein enhanced contextual fear conditioning performance; and (v) baicalein enhanced CREB phosphorylation in rat hippocampal CA1 region.
Memory and cognitive function impairment associated with age-related neurodegenerative diseases, such as brain ischaemia, Alzheimer's disease and Parkinson's disease have become a large public health problem with the increasing elderly population. Considering the relationship between hippocampal LTP and cognition, small molecules that promote LTP in hippocampus could be used as novel agents against age-associated memory impairment. There have been many new drug candidates designed to boost memory in recent years (Marshall, 2004) and flavonoid compounds have received much attention, as they are the most common group of polyphenolic compounds in the human diet and are ubiquitous in plants (Spencer, 2008; 2009; Rendeiro et al., 2009). As a polyphenol that belongs to the flavone subgroup, baicalein has been used to enhance memory for thousands of years in China (Wang et al., 2004b). We have previously reported that baicalein improves learning and memory deficits induced by permanent occlusion of bilateral common carotid arteries of rats (Liu et al., 2007). Furthermore, many studies have shown that baicalein facilitates cognitive functions in the acquisition, consolidation stages of learning and memory processes in the scopolamine-, chronic cerebral hypoperfusion- and β-amyloid peptide-induced dementia of animals (Wang et al., 2004a; He et al., 2009). However, the exact mechanism by which baicalein enhances memory remains unknown.
The hippocampus plays a major role in memory formation and consolidation processes and it is generally accepted that most information is stored at synapses in the form of alterations in synaptic efficiency (Bliss and Collingridge, 1993). In particular, LTP, a form of synaptic plasticity, has been widely used to explore the molecular and cellular basis for learning and memory (Bliss and Collingridge, 1993). Our present results demonstrated that relatively low concentrations of baicalein increased LTP in the hippocampal CA1 region, and this enhancement reached a maximum at a concentration of 1 µM. Unexpectedly, LTP magnitude returned towards the control level when slices were exposed to a higher concentration of this drug. Thus, the dose–response curve for baicalein on LTP showed a bell-shaped feature, and this result was consistent with some previous observations that galantamine (Moriguchi et al., 2009), fisetin (Maher et al., 2006), SKF38393 (Stramiello and Wagner, 2008), and nefiracetam (Moriguchi et al., 2008) potentiate NMDA receptor-dependent LTP in the same bell-shaped manner. Moreover, application of 1 µM baicalein for 30 min did not affect previously induced LTP, suggesting that the drug did not compromise the expression of LTP. A variety of evidence has indicated that increases in both presynaptic release of glutamate and postsynaptic response to glutamate are involved in the expression of LTP (Malenka and Nicoll, 1999). Presynaptic changes can be detected by the PPF technique and a decreased PPR in association with LTP was indicative of an increased probability of presynaptic neurotransmitter release (Schulz et al., 1994). However, we found that 1 µM baicalein did not change the PPR before and after HFS stimulation, suggesting that augmentation of LTP by baicalein did not involve changes in presynaptic neurotransmitter release.
Previous studies have shown that LTP triggered by HFS or short trains of TBS stimulation in hippocampal CA1 area requires postsynaptic molecular mechanisms, such as activation of NMDA receptors and the PI3K signalling pathway (Malenka and Bear, 2004; Raymond, 2007). Such stimulations result in a pattern of glutamate release that is sufficient to activate postsynaptic NMDA receptors and induce NMDA receptor-dependent LTP, which is completely blocked by NMDA receptor antagonists. Consistent with these previous observations, we found that the NMDA receptor antagonists D-APV and MK-801 completely blocked HFS and TBS-induced LTP under our experiment condition. Furthermore, NMDA receptor antagonists totally blocked baicalein-facilitated LTP. Taken together, these results indicate that baicalein promotes NMDA receptor-dependent LTP in hippocampal slices of rats.
The next question should then be which molecule(s) in the postsynaptic neuron is the target of baicalein. Baicalein is known as a 12-LO inhibitor and decreases the generation of 12(S)-HETE and 12(S)-HPETE in cell proliferation studies. Lipoxygenases are non-haem iron proteins and incorporate a molecular oxygen into various positions into arachidonic acid and other polyunsaturated lipids, and there is an expanding literature on the role of arachidonic acid-derived lipids in synaptic plasticity. However, evidence for the role of 12-LO in LTP has been controversial (O'Dell et al., 1991). A recent study using 12-LO knock-out mice indicates that the 12-LO pathway is necessary for the induction of metabotropic glutamate receptor (mGluR)-dependent LTD, but not for NMDA receptor-dependent LTP at CA3-CA1 synapses (Feinmark et al., 2003). Similarly, we found that treatment with 12-HETE and 12-HPETE had no effect on NMDA receptor-dependent LTP. Moreover, the promotion of LTP by baicalein was independent of 12-lipoxygenase inhibition, because 12(S)-HETE and 12(S)-HPETE did not reverse the effect of baicalein. Indeed, many studies have confirmed that a variety of biological activities of baicalein are not associated with 12-LO activity (Suk et al., 2003; Peng et al., 2008; Taniguchi et al., 2008). Regardless of the relevance of 12-LO inhibition in LTP facilitation, baicalein might have inhibited 12-LO activity on brain slices under our experimental conditions. However, NMDA receptor-dependent LTP at CA3-CA1 synapses is not related to 12-LO activity as discussed above. Thus, other molecular mechanisms underlying the effect of baicalein must be investigated.
The PI3K pathway has been classically involved in the regulation of cell growth, survival, proliferation (Brunet et al., 2001). In addition to its well-established role in neuronal survival and differentiation, PI3K is also important in synaptic plasticity and learning and memory. For example, it has been shown that activation of PI3K is required for the expression of LTP in the hippocampal CA1 region (Raymond et al., 2002; Opazo et al., 2003; Racaniello et al., 2010). PI3K may contribute to the regulation of NMDA receptor-dependent LTP and memory formation by facilitating the insertion of AMPA receptors into the postsynaptic membrane (Man et al., 2003). In our previous studies, baicalein attenuated learning and memory deficits and protected neurons against ischaemic injury by activating the PI3K/Akt pathway in rats (Liu et al., 2007; 2010;). Furthermore, other flavonoids such as the citrus flavanone hesperetin activate the PI3K/Akt pathway in neurons and flavonoids are known to activate Akt phosphorylation at Ser473 in a dose-dependent manner (Vauzour et al., 2007; Spencer, 2009). In accordance with a previous report (Opazo et al., 2003), we found here that the PI3K inhibitors LY294002 and wortmannin decreased the magnitude of LTP and PI3K inhibitors completely blocked baicalein-facilitated LTP, supporting an involvement of PI3K signalling in baicalein-facilitated LTP. To determine whether up-regulation of PI3K activity is responsible for the enhancement of LTP by baicalein treatment, we indirectly monitored the activation of PI3K by measuring the phosphorylation of its downstream target Akt at Ser473 using Western blot analysis. We found that HFS induction was associated with an increase in the phosphorylation of Akt at Ser473 time-dependently. Furthermore, increased phosphorylation of Akt was further augmented by baicalein treatment in a bell-shaped dose–response manner that peaked at 1 µM, indicating that the activation of the PI3K pathway by baicalein in hippocampal slices following HFS could account for its enhancement of LTP.
cAMP response element-binding protein is a transcription factor for many genes associated with memory and synaptic plasticity. Furthermore, robust CREB phosphorylation was detected in hippocampus in response to both LTP-inducing stimulation and memory training tasks (Impey et al., 1996; Chen et al., 2005). Various signalling pathways have been linked to CREB activation in the induction of long-lasting changes in synaptic plasticity and memory, including the ERK and PI3K pathways. We found that CREB phosphorylation was significantly increased in the CA1 region of baicalein-treated slices after HFS. Furthermore, baicalein treatment selectively increased the phosphorylation of CREB in the CA1 region of hippocampus, but not in prefrontal cortex, after fear conditioning training. However, there were no significant alterations in ERK phosphorylation in the CA1 region related to baicalein treatment of slices after HFS. These data indicated that baicalein could promote CREB phosphorylation in a PI3K-dependent way, but independent of ERK activity.
It is well established that cued fear conditioning relies on the structural integrity of the amygdala but not the hippocampus, whereas contextual fear conditioning is hippocampus and amygdala-dependent (Anagnostaras et al., 2001; Rodrigues et al., 2004). A number of studies have addressed the role of NMDA receptors in the hippocampus, which is essential for the formation of contextual memory (Kim et al., 1991; Schenberg and Oliveira, 2008; Gao et al., 2009). Dash et al. (2004) found that stimulation of PI3K activity with a synthetic phosphopeptide improved performance in contextual fear conditioning task. In our last set of experiments, given the results above and the known associations between LTP and memory, we examined whether the electrophysiological effects of baicalein seen in hippocampal slices would translate into improvements in memory in normal rats. A previous study of the pharmacokinetics and tissue distribution of baicalein in rats has shown that baicalein rapidly penetrates the blood–brain barrier by 20 min after administration and becomes homogenously distributed over various regions of the brain (brain stem, cerebellum, cerebral cortex, hippocampus, midbrain and striatum) (Tsai et al., 2002). We found that baicalein treatment 20 min before training influenced hippocampus-dependent contextual fear conditioning, but not hippocampus-independent cued fear conditioning, indicating that baicalein may mediate hippocampus-dependent memory but with less effect on amygdala-dependent memory. Moreover, the increase in freezing behaviour in baicalein-treated rats was not due to the changes in locomotive activity and pain sensation, because their response to electric foot shock and the exploratory behaviour during exposure to novel context were similar to those of the control rats.
In summary, the results presented here indicate that baicalein facilitates a postsynaptic NMDA receptor-dependent LTP at the Schaffer collateral-CA1 synapses via stimulation of PI3K activity. We also found that acute administration of baicalein increased hippocampus-dependent contextual fear conditioning performance of rats. These results provide further insight into the mechanisms through which baicalein exerts its beneficial effect on central nervous system disorders and age-associated memory impairment, suggesting that baicalein may be a promising agent for therapy of cognitive deficits associated with neurodegenerative disorders.
Acknowledgments
This work was supported by grants from the Key Project of National Natural Science Foundation of China (NSFC no. 30930104), the National Basic Research Program of China (973 Program, No. 2007CB507404), the Chang Jiang Scholar Program of the Ministry of Education of China to Dr J.G. Chen. It was also supported by the Program for New Century Excellent Talents in Universities of China (NCET-08–0225) to Dr F. Wang and grant from NSFC (No. 30901804) to Dr Z.L. Hu.
Glossary
Abbreviations
- 12(S)-HETE
12(S)-hydroxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid
- 12(S)-HPETE
12(S)-hydroperoxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid
- 12-LO
12-lipoxygenase
- ACSF
artificial cerebrospinal fluid
- CREB
cAMP response element-binding protein
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
- fEPSP
field excitatory postsynaptic potential
- HFS
high-frequency stimulation
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
- PI3K
phosphoinositide 3-kinase
- PPF
paired-pulse facilitation
- PPR
paired-pulse ratio
- TBS
theta-burst stimulation
Conflict of interest
The authors state no conflict of interest.
Supplementary material
Additional Supporting Information may be found in the online version of this article:
Supporting Information: Teaching Materials; Figs 1–8 as PowerPoint slide.
Figure S1 Pre-training administration of baicalein has no effect on cortex-dependent remote memory. (A) Experimental design of fear conditioning paradigm for remote memory test. (B) Total percentage of freezing during the 3 min contextual fear-conditioning test when tested 15 d after fear conditioning training. Groups did not differ in freezing during the cortex-dependent remote memory test compared with controls.
Figure S2 12(S)-HETE and 12(S)-HPETE at various concentrations do not affect baicalein-induced LTP enhancement. (A) Pre-incubation of slices with 12(S)-HETE (100 nM or 1 µM) did not affect the induction of baicalein-facilitated LTP. (B) Histograms indicate the average fEPSP slope after HFS relative to baicalein and the combination of 12(S)-HETE (100 nM or 1 µM) and baicalein. (C) 12(S)-HPETE (100 nM or 1 µM) did not block baicalein-facilitated LTP. (D) Histograms indicate the average fEPSP slope after HFS relative to baicalein and the combination of 12(S)-HPETE (100 nM or 1 µM) and baicalein. The superimposed fEPSP in the top portion showed respective recordings from representative experiments taken at the time indicated by the number. Each point was the normalized mean ± SEM of slices. n = 6.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Jensen V, Hvalby O, Seeburg PH, Kohr G. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 2007;52:77–86. doi: 10.1016/j.neuropharm.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cai F, Wang F, Lin FK, Liu C, Ma LQ, Liu J, et al. Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3beta pathway. Free Radic Biol Med. 2008;45:964–970. doi: 10.1016/j.freeradbiomed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey LA, Imming P. 12-Lipoxygenase: classification, possible therapeutic benefits from inhibition, and inhibitors. Curr Med Chem. 1999;6:389–398. [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moody MR, Moore AN. Performance in long-term memory tasks is augmented by a phosphorylated growth factor receptor fragment. J Neurosci Res. 2004;77:205–216. doi: 10.1002/jnr.20174. [DOI] [PubMed] [Google Scholar]
- Feinmark SJ, Begum R, Tsvetkov E, Goussakov I, Funk CD, Siegelbaum SA, et al. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J Neurosci. 2003;23:11427–11435. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH, et al. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2009;20:1072–1082. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- He XL, Wang YH, Gao M, Li XX, Zhang TT, Du GH. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res. 2009;1249:212–221. doi: 10.1016/j.brainres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009;139:1813S–1817S. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim S, Jeon SJ, Son KH, Lee S, Yoon BH, et al. The effects of acute and repeated oroxylin A treatments on Abeta(25–35)-induced memory impairment in mice. Neuropharmacology. 2008;55:639–647. doi: 10.1016/j.neuropharm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav Neurosci. 1991;105:126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, et al. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta1-42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience. 2009;163:741–749. doi: 10.1016/j.neuroscience.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, et al. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007;86:423–430. doi: 10.1016/j.pbb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112:1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang W, Wang F, Cai F, Hu ZL, Yang YJ, et al. Phosphatidylinositol-linked novel D(1) dopamine receptor facilitates long-term depression in rat hippocampal CA1 synapses. Neuropharmacology. 2009;57:164–171. doi: 10.1016/j.neuropharm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Mans RA, Chowdhury N, Cao D, McMahon LL, Li L. Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience. 2010;166:435–444. doi: 10.1016/j.neuroscience.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall E. Forgetting and remembering. A star-studded search for memory-enhancing drugs. Science. 2004;304:36–38. doi: 10.1126/science.304.5667.36. [DOI] [PubMed] [Google Scholar]
- Morgan SL, Teyler TJ. Electrical stimuli patterned after the theta-rhythm induce multiple forms of LTP. J Neurophysiol. 2001;86:1289–1296. doi: 10.1152/jn.2001.86.3.1289. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Shioda N, Han F, Narahashi T, Fukunaga K. CaM kinase II and protein kinase C activations mediate enhancement of long-term potentiation by nefiracetam in the rat hippocampal CA1 region. J Neurochem. 2008;106:1092–1103. doi: 10.1111/j.1471-4159.2008.05440.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Shioda N, Han F, Yeh JZ, Narahashi T, Fukunaga K. Galantamine enhancement of long-term potentiation is mediated by calcium/calmodulin-dependent protein kinase II and protein kinase C activation. Hippocampus. 2009;19:844–854. doi: 10.1002/hipo.20572. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O'Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Pan SL, Huang YW, Guh JH, Chang YL, Teng CM. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-kappaB activity in vascular smooth-muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:579–588. doi: 10.1007/s00210-008-0328-1. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello M, Cardinale A, Mollinari C, D'Antuono M, De Chiara G, Tancredi V, et al. Phosphorylation changes of CaMKII, ERK1/2, PKB/Akt kinases and CREB activation during early long-term potentiation at Schaffer collateral-CA1 mouse hippocampal synapses. Neurochem Res. 2010;35:239–246. doi: 10.1007/s11064-009-0047-0. [DOI] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the ‘long’ in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ, Crouch MF. The phosphoinositide 3-kinase and p70 S6 kinase regulate long-term potentiation in hippocampal neurons. Neuroscience. 2002;109:531–536. doi: 10.1016/s0306-4522(01)00500-0. [DOI] [PubMed] [Google Scholar]
- Rendeiro C, Spencer JP, Vauzour D, Butler LT, Ellis JA, Williams CM. The impact of flavonoids on spatial memory in rodents: from behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009;4:251–270. doi: 10.1007/s12263-009-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Schenberg EE, Oliveira MG. Effects of pre or posttraining dorsal hippocampus D-AP5 injection on fear conditioning to tone, background, and foreground context. Hippocampus. 2008;18:1089–1093. doi: 10.1002/hipo.20475. [DOI] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP. Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc Nutr Soc. 2008;67:238–252. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–1161. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- Stramiello M, Wagner JJ. D1/5 receptor-mediated enhancement of LTP requires PKA, Src family kinases, and NR2B-containing NMDARs. Neuropharmacology. 2008;55:871–877. doi: 10.1016/j.neuropharm.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk K, Lee H, Kang SS, Cho GJ, Choi WS. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. J Pharmacol Exp Ther. 2003;305:638–645. doi: 10.1124/jpet.102.047373. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Yoshida T, Horinaka M, Yasuda T, Goda AE, Konishi M, et al. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–8927. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY, Chen CF. The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: a microdialysis study. Br J Pharmacol. 2002;137:1314–1320. doi: 10.1038/sj.bjp.0704959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–1367. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- Wang SY, Wang HH, Chi CW, Chen CF, Liao JF. Effects of baicalein on beta-amyloid peptide-(25–35)-induced amnesia in mice. Eur J Pharmacol. 2004a;506:55–61. doi: 10.1016/j.ejphar.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Wang Z, Du Q, Wang F, Liu Z, Li B, Wang A, et al. Microarray analysis of gene expression on herbal glycoside recipes improving deficient ability of spatial learning memory in ischemic mice. J Neurochem. 2004b;88:1406–1415. doi: 10.1046/j.1471-4159.2003.02258.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


