Abstract
BACKGROUND AND PURPOSE
Current data suggest that parathyroid hormone (PTH)-related peptide (PTHrP) domains other than the N-terminal PTH-like domain contribute to its role as an endogenous bone anabolic factor. PTHrP-107-139 inhibits bone resorption, a fact which has precluded an unequivocal demonstration of its possible anabolic action in vivo. We thus sought to characterize the osteogenic effects of this peptide using a mouse model of diabetic low-turnover osteopaenia.
EXPERIMENTAL APPROACH
PTHrP-107-139 was administered to streptozotocin-induced diabetic mice, with or without bone marrow ablation, for 13 days. Osteopaenia was confirmed by dual-energy X-ray absorptiometry and microcomputed tomography analysis. Histological analysis was performed on paraffin-embedded bone tissue sections by haematoxylin/eosin and Masson's staining, and tartrate-resistent acid phosphatase immunohistochemistry. Mouse bone marrow stromal cells and osteoblastic MC3T3-E1 cells were cultured in normal and/or high glucose (HG) medium. Osteogenic and adipogenic markers were assessed by real-time PCR, and PTHrP and the PTH1 receptor protein expression by Western blot analysis.
KEY RESULTS
PTHrP-107-139 reversed the alterations in bone structure and osteoblast function, and also promoted bone healing after marrow ablation without affecting the number of osteoclast-like cells in diabetic mice. This peptide also reversed the high-glucose-induced changes in osteogenic differentiation in both bone marrow stromal cells and the more differentiated MC3T3-E1 cells.
CONCLUSIONS AND IMPLICATIONS
These findings demonstrate that PTHrP-107-139 promotes bone formation in diabetic mice. This mouse model and in vitro cell cultures allowed us to identify various anabolic effects of this peptide in this scenario.
Keywords: C-terminal PTHrP, bone formation, diabetes mellitus, osteopaenia, osteoblastic cells
Introduction
Parathyroid hormone (PTH)-related peptide (PTHrP) is abundant in bone where it seems to act as an important modulator of bone development and bone remodelling (Bisello et al., 2004; Martin, 2005). The N-terminal domain of PTHrP interacts with the PTH1 receptor (PTH1R) in osteoblasts (Dean et al., 2008). As shown to be the case with PTH, this domain is anabolic for bone when administered in an intermittent manner (Rouffet et al., 1994; Stewart et al., 2000; Horwitz et al., 2003; Goltzman, 2008; Lozano et al., 2009). In addition, recent studies strongly suggest that PTHrP domains other than its N-terminal domain might contribute to the PTHrP role(s) in bone. Hence, knock-in mice expressing a truncated form of PTHrP with an intact N-terminal domain but missing both the mid-region [containing the nuclear localization signal (NLS)] and the C-terminal region display premature osteoporosis related to a decrease in bone formation (Miao et al., 2008; Toribio et al., 2010). These studies demonstrate that the NLS and the C-terminus of PTHrP are crucial for the osteogenic commitment and survival of bone marrow cell precursors. In the same line, a recent in vitro study using specific blockade of the latter domains of PTHrP-1-141 supports the idea that the osteogenic action of this protein is not restricted to its N-terminus (Hildreth et al., 2010). However, these experimental approaches do not allow the assignment of these osteogenic effects to either the NLS or the C-terminal domain of PTHrP. Of note in this regard, the C-terminal domain of PTHrP has been shown to be essential for the mitogenic effect induced by the NLS domain in vascular smooth muscle cells (de Miguel et al., 2001). The possibility that such an interaction between both PTHrP domains might modulate bone formation to explain the skeletal phenotype in the aforementioned knock-in mice (Miao et al., 2008) is presently unclear.
Results from both in vitro and in vivo studies strongly suggest that the native C-terminal PTHrP-107-139 fragment can inhibit osteoclast-mediated bone resorption by direct interaction with osteoclasts (Fenton et al., 1991b; Cornish et al., 1997). Possible reasons for some inconsistent results in this respect by some investigators (Murrills et al., 1995) have been attributed to the use of different experimental settings with various osteoclastic cell preparations, as critically discussed by Cornish et al. (1997). The bulk of in vitro studies now indicate that this PTHrP fragment can also directly stimulate osteoblast growth and/or differentiation (Cornish et al., 1999; Valín et al., 2001; Guillén et al., 2002; de Gortázar et al., 2006; Alonso et al., 2008). Some studies have suggested that the N-terminal pentapeptide (osteostatin) might account for at least some of these effects of PTHrP-107-139 in bone cells, through an interaction with an as yet uncharacterized receptor (Fenton et al., 1991a; Cornish et al., 1999; Cuthbertson et al., 1999,Valín et al., 2001; Lozano et al., 2010). We recently reported that PTHrP-107-139 reversed at least in part the deleterious effects of 3-methylprednisolone on bone formation in mice (de Castro et al., 2010). However, neither of the aforementioned findings rules out a putative involvement of bone resorption inhibition in the observed osteogenic effects of this C-terminal PTHrP domain. Hence, current data do not unequivocally establish the role of PTHrP-107-139 as a bone anabolic factor that can promote bone formation in vivo.
Studies in humans and rodents demonstrate that a low bone mineral density (BMD) occurs in type 1 diabetes mellitus, associated with decreased bone formation by ill-defined mechanisms. In addition, the results from most of these studies evaluating osteoclast activity by histology and/or bone turnover markers strongly suggest that bone resorption is also suppressed in this type of diabetes (Inzerillo and Epstein, 2004; Hamada et al., 2007; Hofbauer et al., 2007; McCabe, 2007; Silva et al., 2009). Therefore, in contrast to type 2 diabetic states in which a deterioration of bone integrity is apparently related to decreased bone formation and increased osteoclastogenesis (Inzerillo and Epstein, 2004; Kawashima et al., 2009; Nuche-Berenguer et al., 2009; 2010;), type 1 diabetes-related osteopaenia exhibits features consistent with a low bone turnover. We recently identified PTHrP down-regulation as an underlying mechanism contributing to osteopaenia in streptozotozin (STZ)-induced type 1 diabetic mice (Lozano et al., 2009). On the other hand, recent studies in noninsulin-dependent diabetic patients showed higher circulating PTHrP levels which directly correlate with glycaemia and calcaemia, as compared with control subjects (Suzuki et al., 2000; Legakis and Mantouridis, 2009). The true roles of the various forms of PTHrP in states of osteopaenia, such as occurs in diabetes, require further investigation.
Here, we aimed to assess the efficacy of PTHrP-107-139 in preventing bone loss and promoting bone regeneration in well-characterized STZ-induced diabetic mice exhibiting low bone turnover (Botolin et al., 2005; Hamada et al., 2007). Using these mice as well as an in vitro approach, we designed this study to disclose the effects of this C-terminal PTHrP domain on bone formation, independently of its reported osteoclast inhibitory action.
Methods
Diabetic mouse model
Male CD-1 mice (11 weeks old) from Harlan Laboratories (Harlan Interfauna Ibérica, Barcelona, Spain) were stabilized in the Animal Research Facility at Fundación Jiménez Díaz for 2 weeks. Animals were then weighed, and injected i.p. with STZ (45 µg·g−1 body weight in 50 mM sodium citrate buffer, pH 4.5) or buffer alone (controls) for 5 days. Seven days after the last injection, non-fasting glucose was tested in blood drawn from the mouse tail using a glucometer (Glucocard G± meter, A. Menarini Diagnostics, Firenze, Italy). Experimental procedures were started 2 weeks after confirmation of diabetes (blood glucose >3 g·L−1). Animals were allowed free access to water and fed a standard diet (8.8 g·Kg−1 calcium and 5.9 g·Kg−1 phosphate; Panlab, Reus, Spain) in a room maintained at 22°C on 12 h light/12 h dark cycles.
At the beginning of the study, mice were randomly divided into three groups: control mice (n= 5) and two groups of diabetic mice, which were administered PTHrP-107-139 (100 µg·Kg−1every other day, s.c.) (n= 5) or vehicle (50 mM KCl, pH 4.5) (n= 10) for 13 days. On day 8 of this treatment, mice were anaesthetized with ketamine/xylazine (2:1, v·v−1), and bone marrow ablation was performed in both tibiae as described previously (Lozano et al., 2009). On day 14, 24 h after the last injection of PTHrP-107-139 or its vehicle, all mice were killed by cervical dislocation. The ablated tibiae and intact femurs from each animal were harvested and freed from soft tissues. Both tibiae and one femur were assigned to histological evaluation and/or total RNA extraction, and the other femur was sent to Trabeculae®, Empresa de Base Tecnológica, S.L., for microcomputed tomography (µCT) evaluation.
Two other groups of diabetic mice, treated or not with PTHrP-107-139 as above but not undergoing bone marrow ablation, and a group of control mice (n= 5 per experimental group) were assigned to bone marrow cell (BMC) cultures as described below. Studies were performed with the approval of the Institutional Animal Care and Use Committee at Fundación Jiménez Díaz, according to the European Union guidelines.
Histological analysis
One ablated tibia was fixed in 4% p-formaldehyde in phosphate-buffered saline and subsequently decalcified in Mielodec (Bio-Optica, Milan, Italy), dehydrated and embedded in paraffin. Histological analysis was carried out by haematoxylin/eosin and Masson's staining of two sagittal 4 µm sections from each mouse in five mice per experimental group. The % area of each examined field containing connective tissue and the number of osteoblasts (cuboidal cells on newly formed bone and endosteal surfaces) were assessed within an ablated 2.8 mm2 area from the growth plate using Image-Pro Plus 5.0 (Media Cybernetics, Silver-Spring, MD, USA). Blood vessels were identified in this area by the presence of red blood cells in their lumen, and confirmed with lectin staining (Lozano et al., 2009; de Castro et al., 2010). Adipocytes were identified as unstained round spaces within the marrow in an area between mid-diaphysis and distal metaphysis and quantified.
Tartrate-resistant acid phosphatase (TRAP) was detected in bone tissue samples by immunohistochemistry using a specific rabbit polyclonal antibody (H00000054-D01P, Abnova, Jhongli City, Taiwan), as recently described (de Castro et al., 2010). TRAP staining has been traditionally used as a marker for mature polynucleated osteoclasts (Jia et al., 2006; Kim et al., 2006; de Castro et al., 2010), but it has also been detected in mononuclear phagocytes including osteoclast precursors in the bone marrow (Connor et al., 1995; Hayman et al., 2001). Polynuclear osteoclast-like cells (with three or more nuclei) and mononuclear cells showing TRAP positivity were identified and counted in the same regenerating area used to evaluate the number of osteoblasts at the proximal methaphysis, using the same magnification (×400). Evaluations were performed by three independent observers in a blinded fashion, and the corresponding mean score value was obtained for each mouse.
Dual-energy X-ray absorptiometry (DXA) and µCT analysis
Bone mineral density (BMD) and bone mineral content (BMC) of one intact femur were measured in anaesthetized control and diabetic mice at the start of the study and at the end of PTHrP-107-139 (or vehicle) treatment by DXA using PIXImus (GE Lunar Corp., Madison, WI, USA) (Lozano et al., 2009). The other femur was scanned with a high-resolution microtomographic system (SkyScan 1172, Skyscan N.V., Aartselaar, Belgium) at Trabeculae®. The three-dimensional microstructural properties of the trabecular bone region of interest in the metaphysis were assessed by using SkyScan™ CT-analyzer software, version 1.7.0.5 (de Castro et al., 2010). The following parameters were calculated: % bone volume, bone surface, trabecular thickness, trabecular number, structure model index, trabecular bone pattern factor and degree of anisotropy.
Cell culture studies
Bone marrow stromal cells (BMSCs) were prepared from intact mouse tibiae and femurs following a previously published protocol (Lozano et al., 2009; de Castro et al., 2010). BMSCs in α-minimum essential medium (α-MEM) containing 15% FBS, 1% penicillin–streptomycin and 2.5 µg·mL−1 fungizone were seeded at 1–2.5 × 106 cells·cm−2 onto six-well plates in 5% CO2 at 37°C. Osteogenic medium (the aforementioned medium with 50 µg·mL−1 ascorbic acid and 10 mM β-glycerophosphate) was added at day 3. Half of the volume of the cell-conditioned medium was exchanged every other day. On day 15, cell colonies were fixed with 75% ethanol, and stained for alkaline phosphatase (ALP) activity using naphtyl AS-MX phosphate as substrate and fast blue salt as the coupling chromogen. Total cell number was assessed with crystal violet stain, eluted with 0.2% Triton X-100 and measuring absorbance at 540 nm. Matrix mineralization was determined at day 21 of culture by staining with 40 mM alizarin red S, pH 4.2, for 10 min. Area containing ALP+ colonies and mineralized nodules were quantified using Photoshop cs 8.0.1. (Adobe Systems Inc., San Jose, CA, USA) The diameter of ALP+ colonies was also determined.
BMSCs from control mice (n= 8) were grown onto six-well plates in osteogenic or adipogenic (α-MEM, 15% FBS, 1% penicillin–streptomycin and 2.5 µg·mL−1 troglizatone) medium with normal (5.5 mM) or high glucose (25 mM), in the presence or absence of PTHrP-107-139 (100 nM) for the first 6 h of each consecutive 48 h incubation cycle or continuously for 15 or 21 days. Culture medium was replaced every 48 h. On day 15, total cell RNA was extracted (see below) and total and ALP+ colonies were evaluated as described above. Adipocyte formation was determined in 4%-formaldehyde- fixed cells after Oil Red-O staining. Oil Red-O reagent was prepared by diluting a stock solution (0.3 g of Oil Red-O in 100 mL of isopropanol) with distilled water (6:4, v·v−1) followed by filtration. The number of formed adipocytes was counted in six fields within each plate well under microscope, and the mean was calculated to yield the number of adipocytes per field. Matrix mineralization was assessed on day 21, as described above.
Mouse osteoblastic MC3T3-E1 cells (kindly provided by C. Zaragoza, PhD., Centro Nacional de Investigaciones Cardiológicas, Madrid, Spain) were plated at 20 000 cells·cm−2 and grown in α-MEM with 10% FBS and 1% penicillin–streptomycin with the differentiation-promoting supplements as described above for 5 days, with or without high glucose (25 mM) (or manitol as osmotic control), supplemented (or not) with the test PTHrP peptides. In some experiments, neutralizing rabbit polyclonal antiserum C7 recognizing the osteostatin epitope in PTHrP (107-139) (Valín et al., 1997) at 1:100 dilution, the PTH1R antagonist, [Asn10, Leu11, D-Trp12] PTHrP-7-34 amide [PTHrP-7-34] at 1 µM or PTHrP-109-138 at 100 nM, were added to normal glucose medium. Medium and different stimuli were replaced every other day.
Real-time PCR
Total RNA was extracted from bone samples, which were crushed under liquid nitrogen and cell cultures (48 h after the last treatment with stimuli) by using Trizol (Invitrogen, Groningen, the Netherlands). cDNA synthesis was performed using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI, USA) with random hexamer primers, and real-time PCR was carried out in an ABI PRISM 7500 system (Applied Biosystems, Foster City, CA, USA). Unlabelled mouse-specific primers for: Runx2, osterix, osteocalcin (OC), PTH1R, osteoprotegerin (OPG) and receptor activator of a nuclear factor-κβ ligand (TNFRSF11A) (osteoblastic genes); peroxisome proliferator-activated receptor (PPARγ2) and adipocyte fatty acid–binding protein (FABP4) (adipocyte genes); and vascular endothelial growth factor (VEGF) and its receptors (VEGFR-1 and -2), and TaqMan MGB probes were obtained by Assay-by-DesignSM (Applied Biosystems). Results are expressed in mRNA copy numbers, calculated for each sample using the cycle threshold (Ct) value and normalized against 18S rRNA.
Western blot analysis
MC3T3-E1 cell extracts were obtained in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS, 1 mM phenylmethylsulphonylfluoride and 0.8 µM aprotinin). Protein content was determined by Bradford's method (Pierce, Rockford, IL, USA), using bovine serum albumin as standard. Cell protein extracts (50 µg protein) were transferred onto nitrocellulose membranes (GE Healthcare, Buckinghamshire, UK), blocked with 5% defatted milk in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl with 0.05% Tween-20, and then incubated overnight at 4°C with either rabbit polyclonal antibody to PTH1R (Ab-IV, Covance, Berkeley, CA, USA) at 1:1000 dilution, or rabbit polyclonal anti-PTHrP antiserum C6 at 1:2500 dilution (Izquierdo et al., 2006). The membranes were subsequently incubated with peroxidase-conjugated goat anti-rabbit antibody for 1 h at room temperature. As a loading control, an anti-β-actin antibody was used. Bands were developed by ECL chemiluminiscence (GE Healthcare), and fluorogram bands were quantified by densitometry.
We followed the drug/molecular target nomenclature guidelines according to Alexander et al. (2009) throughout the text.
Statistical analysis
All of the data are expressed as mean ± SEM. Comparisons were performed by Kruskal–Wallis non-parametric analysis of variance followed by Dunn's post hoc test or Mann–Whitney test, as appropriate. P < 0.05 was considered significant.
Results
PTHrP-107-139 administration reverses the alterations in bone mass, trabecular structure and bone-related genes in the diabetic mouse femur
As expected, at the end of the period of study, STZ-injected mice were hyperglycaemic (5170 ± 10 mg·L−1 vs. 1230 ± 30 mg·L−1 in controls; P < 0.01), and showed a significant decrease in body weight as compared with control mice (38.4 ± 0.8 vs. 43.2 ± 1.2 in the control group; P < 0.05) (mean ± SEM). In addition, BMD and BMC values were significantly lower in the femur of diabetic mice than in their controls (Figure 1A). Furthermore, µCT analysis showed that % bone volume, bone surface, trabecular thickness and trabecular number were all decreased in the femoral metaphysis of the diabetic animals, as compared with their controls (Table 1). Moreover, degree of anisotropy and structure model index, representing a measurement of preferential alignment of trabecular structures and surface convexity, respectively, and trabecular bone pattern factor, an estimation of trabecular connectivity, were also significantly altered at this bone site in diabetic mice (Table 1). Alterations in the distal femoral metaphysis of these mice are clearly depicted by µCT images (Figure 1B). Treatment with PTHrP-107-139 was shown to reverse, at least in part, these diabetes-related changes in bone structure in these animals (Figure 1A and B and Table 1). Consistent with these findings, PTHrP-107-139 administration also induced significant changes in several bone genes promoting osteogenesis in the intact femur of diabetic mice (Figure 1C).
Figure 1.
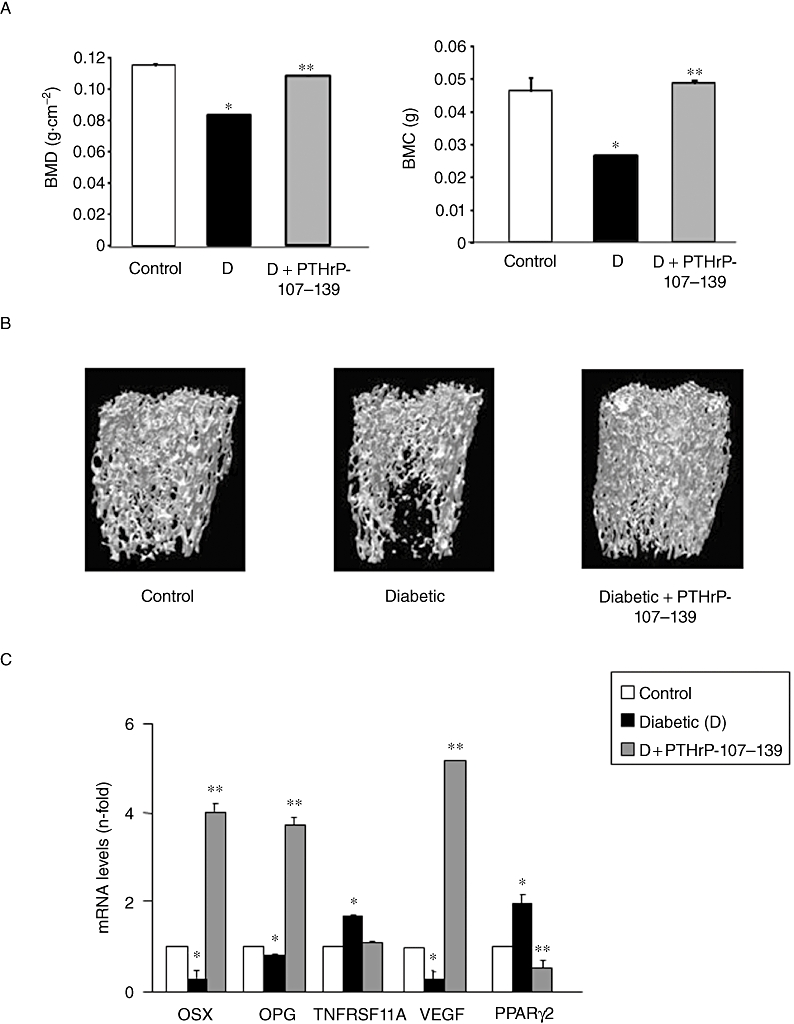
Bone mineral density (BMD) and bone marrow cells (BMC) values (A) and representative microcomputed tomography images of trabecular areas of the intact distal femur (B) in control and diabetic mice, treated or untreated with parathyroid hormone-related protein (PTHrP)-107-139 as described in the text. (C) Changes in the gene expression of several bone-related factors (assessed by real-time PCR) in the intact femur from these mice. Results are mean ± SEM of five control mice and 15 diabetic mice; five of the latter were treated with the PTHrP peptide. *P < 0.05 versus corresponding control value; **P < 0.05 versus corresponding diabetic value. OPG, osteoprotegerin; OSX, osterix; PPARγ2, peroxisome proliferator-activated receptor; TNFRSF11A, receptor activator of nuclear factor-κβ ligand; VEGF, vascular endothelial growth factor.
Table 1.
Trabecular bone structure parameters in the femur metaphysis from control and diabetic mice, treated or not with parathyroid hormone-related protein (PTHrP)-107-139
| Control | Diabetic | Diabetic + PTHrP-107-139 | |
|---|---|---|---|
| BV/TV (%) | 16.85 ± 0.62 | 10.25 ± 0.90* | 12.43 ± 1.00*** |
| BS (mm2) | 65.41 ± 3.85 | 42.24 ± 4.1** | 51.93 ± 4.55*** |
| Tb.Th (mm) | 0.071 ± 0.003 | 0.060 ± 0.001** | 0.060 ± 0.003 |
| Tb.N (mm−1) | 2.66 ± 0.11 | 1.54 ± 0.16** | 2.43 ± 0.18*** |
| Tb.Pf (mm−1) | 15.05 ± 0.39 | 24.29 ± 1.69** | 20.07 ± 1.07*** |
| SMI | 1.62 ± 0.02 | 2.12 ± 0.05* | 1.81 ± 0.05*** |
| DA | 3.50 ± 0.25 | 2.37 ± 0.14* | 2.75 ± 0.03*** |
BS, bone surface; BV/TV, trabecular bone volume/tissue volume; DA, degree of anisotropy; SMI, structure model index; Tb.N, trabecular number; Tb.Pf, trabecular bone pattern factor; Tb.Th, trabecular thickness. Values are mean ± SEM of five animals per group.
P < 0.05;
P < 0.01 versus control;
P < 0.05 versus diabetic.
Treatment with PTHrP-107-139 promotes bone regeneration after marrow ablation in diabetic mice
We also examined whether administration of PTHrP-107-139 might induce intramembranous bone formation following marrow ablation in the tibia of diabetic mice, a process that is hampered in this setting (Lu et al., 2003). We found that the diabetes-related decrease in both regenerating bone formation and osteoblast number in the mouse tibial metaphysis was reversed by PTHrP-107-139 (Figure 2A and B). In contrast, this peptide failed to modify the reduced number of polynucleated TRAP-positive osteoclast-like cells at the expense of more mononuclear TRAP-positive cells in this diabetic model (Figure 2D). On the other hand, PTHrP-107-139 administration prevented the increase in the number of adipocytes in the regenerating tibia from diabetic mice (Figure 3A). Consistent with these histological changes, this peptide also reversed the diabetes-related decrease in the expression of several osteoblastic differentiation genes (Figure 2C) and the increase in FABP4 and PPARγ2 gene expression (Figure 3B). This was associated with an accompanying stimulating effect of PTHrP-107-139 on both the number of blood vessels and the VEGF system in the regenerating mouse tibia (Figure 3C and D).
Figure 2.
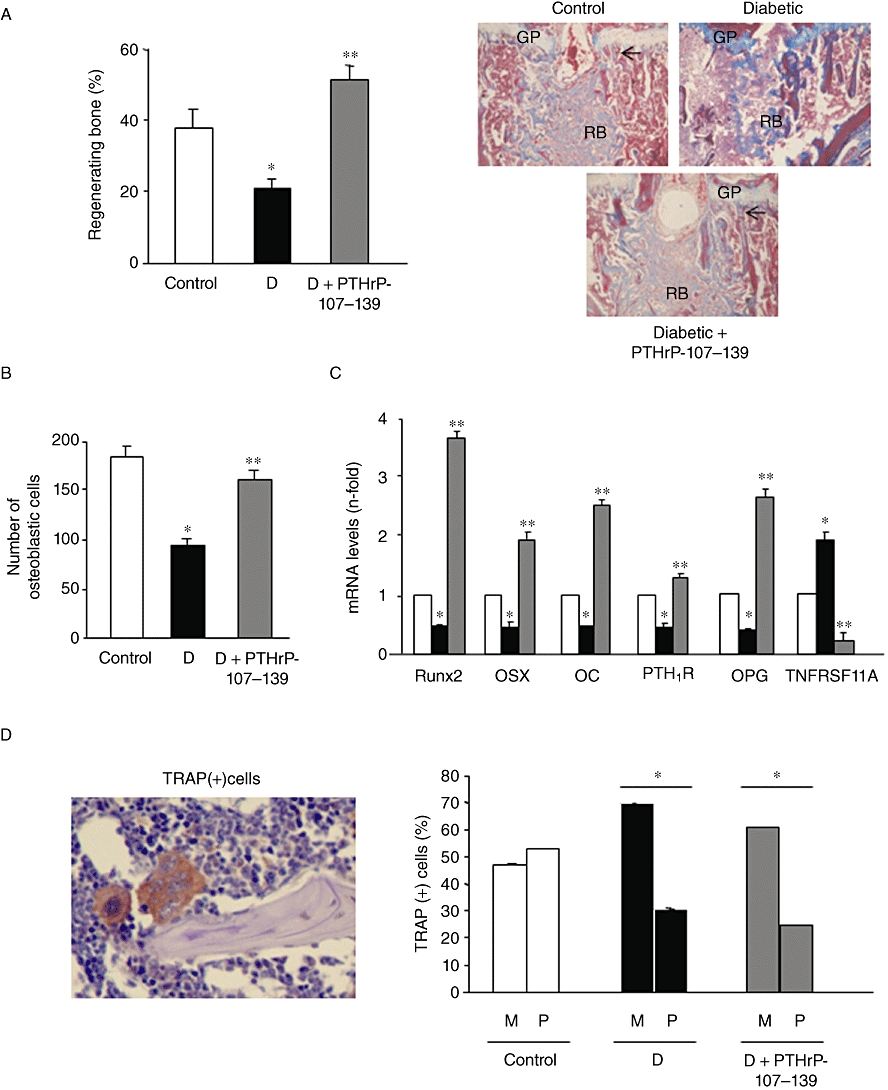
Effect of parathyroid hormone-related protein (PTHrP)-107-139 administration on several diabetes-related alterations in the mouse tibia after marrow ablation: regenerating bone formation (assessed by Masson's staining) (A); abundance of osteoblasts (B); gene expression of various bone-related factors (assessed by real-time PCR) (C); as well as polynucleated osteoclast-like (P) and mononuclear (M) tartrate-resistant acid phosphatise (TRAP)-positive cells (D). For details, see Methods. Representative images are shown in (A) and (D) (original magnifications ×40). Results are mean ± SEM corresponding to the groups of mice as mentioned in the legend to Figure 1. *P < 0.05 versus corresponding control value; **P < 0.05 versus corresponding diabetic value. Arrows denote osteoblast presence. GP, growth plate; OC, osteocalcin; OPG, osteoprotegerin; OSX, osterix; TNFRSF11A, receptor activator of nuclear factor-κβ ligand; RB, regenerating bone.
Figure 3.
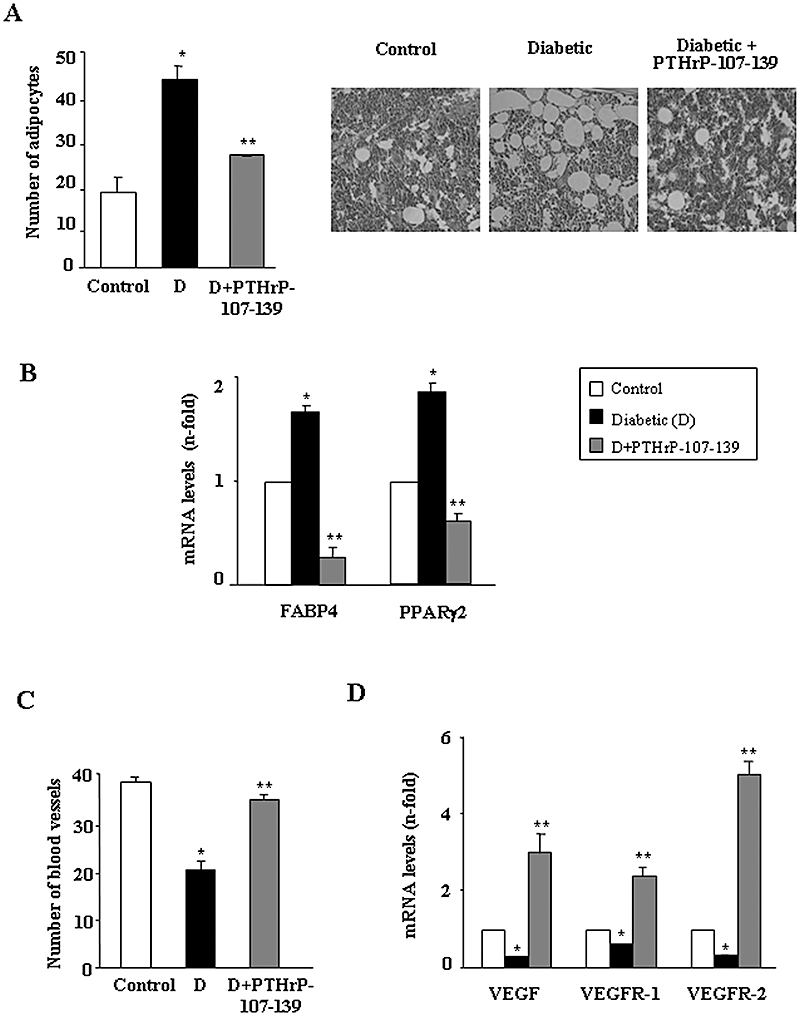
Treatment with parathyroid hormone-related protein (PTHrP)-107-139 reverses the diabetes-induced changes in: the number of adipocytes. Representative images are shown (original magnifications ×40) (A); gene expression of adipogenesis-related factors (assessed by real-time PCR) (B); the abundance of blood vessels (C); and the vascular endothelial growth factor (VEGF) system mRNA levels (D) in the regenerating mouse tibia. Results are mean ± SEM corresponding to the groups of mice as mentioned in the legend to Figure 1. *P < 0.05 versus corresponding control value; **P < 0.05 versus corresponding diabetic value. FABP, adipocyte fatty acid–binding protein; PPARγ2, peroxisome proliferator-activated receptor; VEGFR, VEGF receptor.
PTHrP-107-139 exerts osteogenic effects in vivo and in vitro in mouse BMSCs in a diabetic setting
These observed osteogenic effects of PTHrP-107-139 in vivo were further confirmed using ex vivo BMSC cultures. In vivo administration of this peptide was found to reverse, at least in part, the decrease in ALP+ area (Figure 4A) and the abundance of small ALP+ colonies (Figure 4B), related to a diminished total colony formation (Figure 4C), and diminished area of mineralized nodules (Figure 4D) in BMSCs from diabetic mice as compared with those from controls. Furthermore, we evaluated whether PTHrP-107-139 treatment in vitro of control mouse BMSCs grown in a high-glucose environment also exhibited these bone anabolic features. We found that PTHrP-107-139, at 100 nM, reversed the deleterious changes induced by high glucose in total colony formation and osteogenic differentiation (based on ALP+ colony formation and mineralization) in these cell cultures (Figure 5A–D). This peptide treatment also reversed the changes in the expression of osteoblast (Runx2, OC and PTH1R) and adipocyte (FABP4 and PPARγ2) differentiation markers triggered by high glucose in these cultures (Figure 6A). Moreover, the adipogenesis-promoting effect of high glucose, as observed by growing BMSCs in an adipogenic medium, was significantly inhibited by PTHrP-107-139 (Figure 6B). Interestingly, 6 h intermittent exposure of these cells to this peptide was similarly effective in this respect (Figures 5 and 6).
Figure 4.
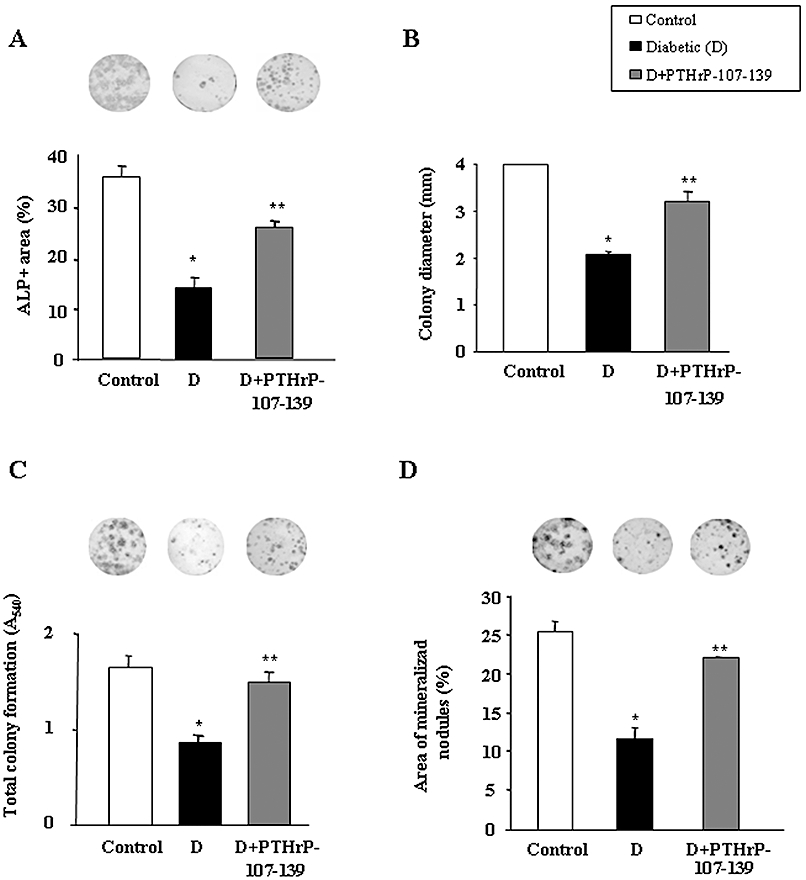
Effect of parathyroid hormone-related protein (PTHrP)-107-139 administration in vivo on the diabetes-related changes in osteogenic differentiation of mouse bone marrow cells (BMC) cultures ex vivo. Changes in alkaline phosphatise (ALP)+ colonies (positive area and colony size distribution) (A and B), total colony formation (assessed by crystal violet staining) (C) and matrix mineralization (evaluated by alizarin red staining) (D) were determined at 15 or 21 days of culture, as described in the text. Representative images of ALP+ (A) and total (C) colonies, and mineralized nodules (D) are shown. BMC cultures from each five mice per experimental group were independently analysed, and the results were then combined for statistical analysis. Results are mean ± SEM. *P < 0.05 versus corresponding control value; **P < 0.05 versus corresponding diabetic value.
Figure 5.
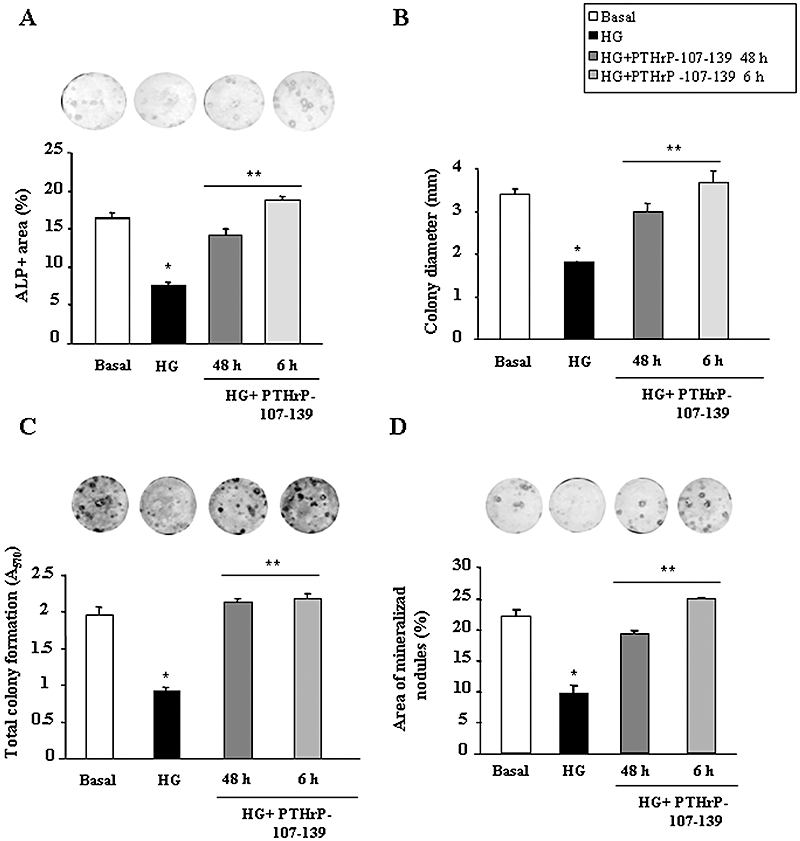
Effects of parathyroid hormone-related protein (PTHrP)-107-139 on high glucose (HG)-induced changes in osteogenic differentiation of mouse bone marrow cells (BMC) cultures in vitro. BMCs were grown in osteogenic medium, with or without (basal) HG, and PTHrP-107-139 (100 nM) (or vehicle), intermittently for only the first 6 h of each consecutive 48-h incubation cycle or continuously (every 48 h), for 15 or 21 days of culture. Changes in alkaline phosphatise (ALP)+ colonies (positive area and colony size distribution) (A and B), total colony formation (assessed by crystal violet staining) (C) and matrix mineralization (evaluated by alizarin red staining) (D) were determined at 15 or 21 days of culture, as described in the text. Representative images of ALP+ (A) and total (C) colonies, and mineralized nodules (D) are shown. BMC cultures from four mice were independently analysed, and the results for each parameter tested were then combined for statistical analysis. Results are mean ± SEM. *P < 0.05 versus corresponding basal value; **P < 0.05 versus corresponding HG value.
Figure 6.
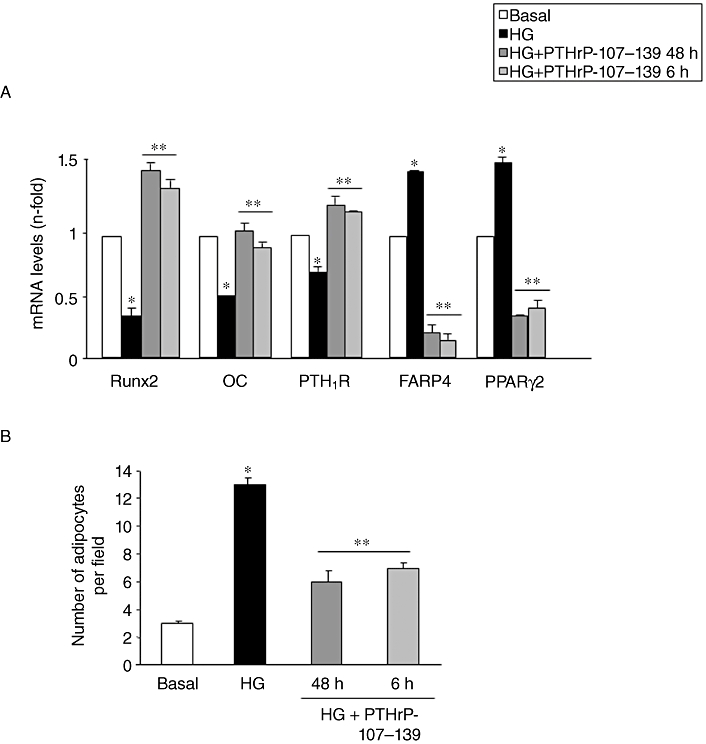
Changes in gene expression of several osteoblastic and adipocytic factors (assessed by real-time PCR) in mouse bone marrow cells (BMC) cultures grown in osteogenic medium, in the presence or absence (basal) of high glucose (HG), with or without parathyroid hormone-related protein (PTHrP)-107-139 (100 nM) as described in the legend to Figure 5 for 15 days (A). Changes in adipocyte formation in BMC cultures in adipogenic medium during this time period were evaluated in these experimental conditions (B). BMC cultures from 4 mice were independently analysed, and the results were then combined for statistical analysis. Results *P < 0.05 versus corresponding basal value; **P < 0.05 versus corresponding HG value. FABP, adipocyte fatty acid–binding protein; OC, osteocalcin; PPARγ2, peroxisome proliferator-activated receptor; PTH1R, parathyroid hormone receptor 1.
PTHrP-107-139 increases osteoblast maturation in mouse MC3T3-E1 cells
We previously reported that high glucose, through an osmotic stress-dependent mechanism, decreases osteoblastic maturation in MC3T3-E1 cells (Lozano et al., 2009), representing a more differentiated osteoblastic phenotype than BMCs (Chen et al., 2002, 2004; Barbara et al., 2004). This effect is related to a down-regulation of the PTHrP/PTH1R system at the gene level and, as confirmed here, at the protein level (Figure 7A). Therefore, we further examined the osteogenic effects of PTHrP-107-139 in MC3T3-E1 cells. Addition of PTHrP-107-139, at 100 nM, to the high glucose medium prevented the alterations in several genes related to osteoblastic maturation in these cells (Figure 7B). This stimulating effect of PTHrP-107-139 on OPG gene expression was concentration-dependent, occurring even at 10 pM (Figure 7C). As shown in Figure 7D, exposure of MC3T3-E1 cells to neutralizing C-terminal PTHrP antiserum C7 in normal glucose medium decreased the expression of several osteoblast differentiation genes. On the other hand, PTHrP-107-139, at 100 nM, but not PTHrP-109-138 as expected (Fenton et al., 1991a), stimulated these genes; and this effect was inhibited by the aforementioned antiserum (Figure 7D).
Figure 7.
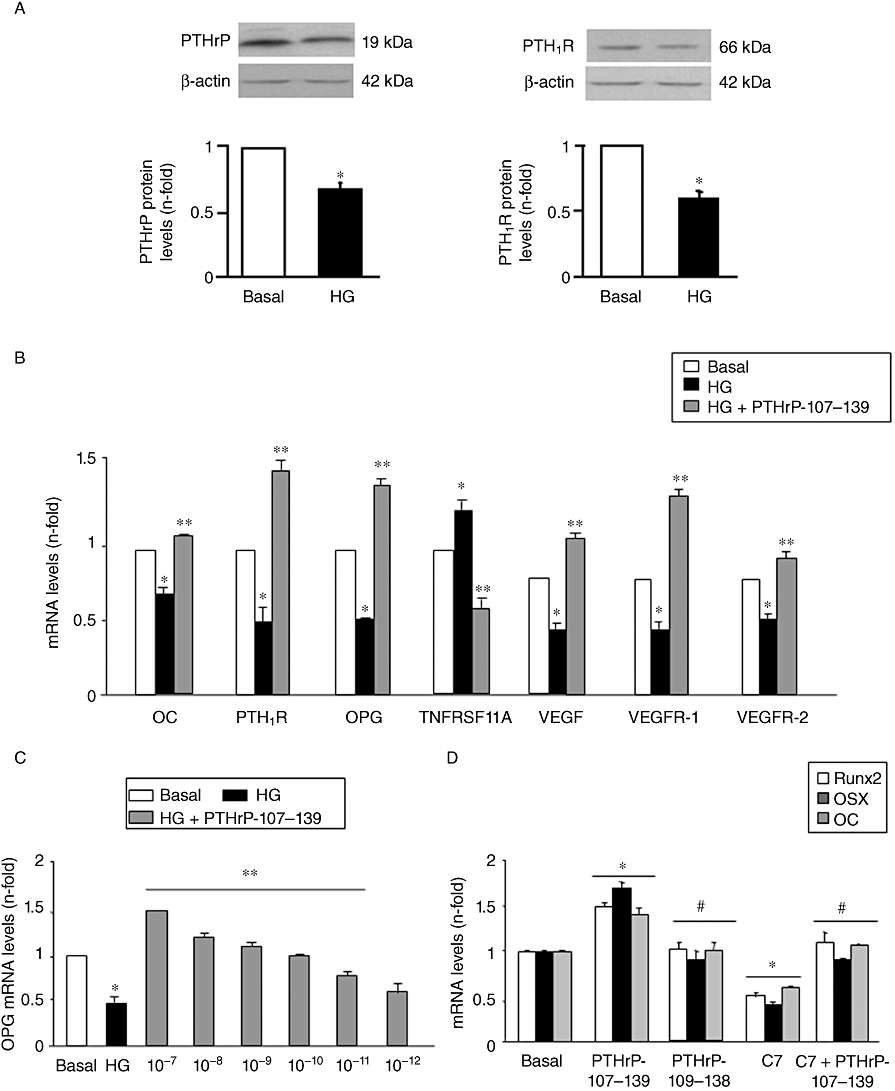
Changes in parathyroid hormone-related protein (PTHrP) and parathyroid hormone receptor 1 (PTH1R) protein expression (assessed by Western blot; representative autoradiograms are shown) (A), and in gene expression of various bone-related factors [assessed by real-time PCR] (B and C) induced by high glucose (HG), with or without PTHrP-107-139, at 100 nM (B) or at different concentrations (C), for 5 days in MC3T3-E1 cells. Opposing effects of PTHrP-107-139 and a neutralizing C-terminal PTHrP antiserum on the expression of osteoblast differentiation genes (assessed by real-time PCR) in these cells grown for 5 days in normal glucose medium. PTHrP-107-139 and PTHrP-109-138 were added at 100 nM, and antiserum C7 was used at 1:100 dilution (D). Results are mean ± SEM of 3–5 independent experiments in duplicate. *P < 0.05 versus corresponding basal value; **P < 0.05 versus corresponding HG value; #P < 0.05 versus PTHrP-107-139 value. OC, osteocalcin; OPG, osteoprotegerin; TNFRSF11A, receptor activator of nuclear factor-κβ ligand; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Recently, we demonstrated that the N-terminal fragment of PTHrP induced very similar effects to those triggered by PTHrP-107-139, as reported here, on osteoblastic differentiation in MC3T3-E1 cells grown in high glucose (Lozano et al., 2009). Thus, we tested whether these combined fragments might have additive effects in this regard. We found that addition of both PTHrP-1-36 and PTHrP-107-139 together, at a submaximal concentration (1 nM) inducing OPG gene overexpression, failed to elicit a greater effect than that of each peptide alone in these cells (Figure 8A). The putative interaction between both PTHrP peptides to modulate osteoblastic function seems to occur downstream from PTH1R, because the PTHrP-107-139-induced OC gene up-regulation was similar in magnitude in the presence or absence of the PTH1R antagonist, PTHrP-7-34, at 1 µM, in MC3T3-E1 cells (Figure 8B).
Figure 8.
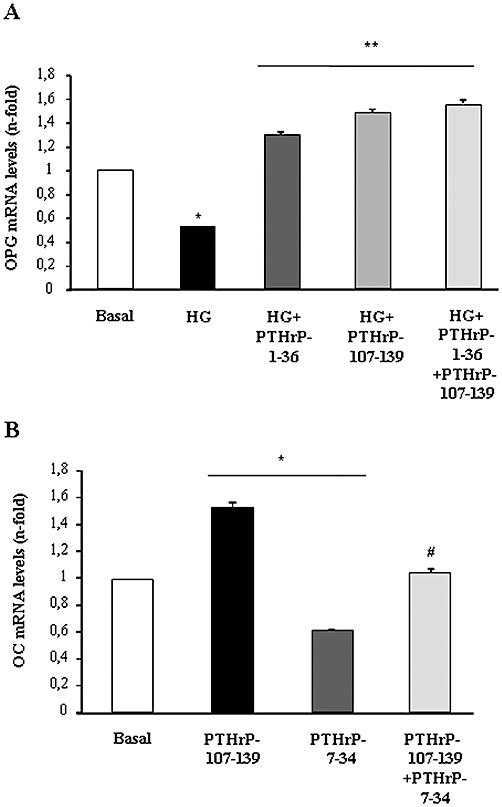
The presence of parathyroid hormone-related protein (PTHrP)-1-36 or the PTH1R antagonist PTHrP-7-34 does not affect the osteogenic effect of PTHrP-107-139 in MC3T3-E1 cells. Cells were grown for 5 days in high glucose (HG) medium (A) or normal glucose medium (B) in the presence or absence of PTHrP-1-36 and PTHrP-107-139 or both (at 1 nM each) (A), or PTHrP-107-139 (100 nM) with or without PTHrP-7-34 (1 µM) (B). Osteoprotegerin (OPG) and osteocalcin (OC) mRNA expression was assessed by real-time PCR. Results are mean ± SEM of three independent experiments in duplicate. *P < 0.05 versus corresponding basal value; **P < 0.05 versus corresponding HG value; #P < 0.05 versus PTHrP-7-34 value.
Discussion and conclusions
In the present study, treatment with PTHrP-107-139 for 13 days was shown to exert a clear osteogenic action (as assessed by DXA, µCT, gene expression analysis and ex vivo BMSCs) in the intact long bones of diabetic mice. Furthermore, our findings herein demonstrate that this treatment promotes intramembranous bone formation following marrow ablation in these mice. Our results showing trabecular bone loss related to a deficient osteoblast function in STZ-induced diabetic mice confirm those previously reported in this animal model, which has proven to be a valid model for studying the mechanisms of diabetes-related osteopaenia (Lu et al., 2003; Botolin et al., 2005; Botolin and McCabe, 2007; Hamada et al., 2007; Lozano et al., 2009; Silva et al., 2009). In fact, the reported lack of efficacy of STZ to affect adipogenic or osteogenic differentiation of mesenchymal C3H10T1/2 cells rules out the possibility that a direct effect of this drug on bone cells might contribute to bone alterations in this model (Botolin et al., 2005; Lozano et al., 2009).
The present data also show an angiogenic effect of PTHrP-107-139, related to its effect on VEGF and its receptors 1 and 2 in the diabetic mice. In this regard, PTHrP-107-139 has previously been shown to stimulate both VEGF and VEGFR-2 expression in human osteoblastic cells in vitro, and also in the regenerating tibia of mice undergoing glucocorticoid treatment (Esbrit et al., 2000; de Gortázar et al., 2006; Alonso et al., 2008; de Castro et al., 2010). The up-regulation of both VEGFR-1 and -2 as found here is of pathophysiological significance considering recent findings showing that both receptors contribute to both neoangiogenesis and new bone formation in a murine model of distraction osteogenesis (Jacobsen et al., 2008). Our data further support the idea that the VEGF system has an important role in the osteogenic action of the C-terminal domain of PTHrP.
Even though dynamic histomorphometry to directly assess the bone formation rate was not performed in the present study, the observed osteogenic effects of PTHrP-107-139 in STZ-induced diabetic mice are likely to be a consequence of its anabolic action and independent of its antiresorptive properties. Thus, TRAP-positive polynucleated osteoclast-like cells were scarce in the regenerating tibia of the diabetic mice at the time of study, in agreement with previous observations in these marrow-ablated mice (Okuda et al., 2007) and in this diabetic model (Hamada et al., 2007; McCabe, 2007; Silva et al., 2009), and their number was unaffected by PTHrP-107-139 treatment. This seemingly low abundance of osteoclasts seems to be somewhat inconsistent with the increased gene expression of TNFRSF11A in the long bones of diabetic mice. However, this apparent discrepancy might be explained by recent in vitro findings showing that chronic exposure of murine osteoclast precursors to high glucose inhibits TNFRSF11A-induced osteoclastogenesis and osteoclast function through a redox mechanism (Wittrant et al., 2008).
Previous studies have assessed the putative osteogenic effects of daily administration of either PTHrP-107-139 or osteostatin, at the same molar concentration and a similar time period (≤2 weeks) as used here, in mice undergoing glucocorticoid treatment or ovariectomized rats respectively. Both C-terminal PTHrP peptides were found to compensate the osteopaenia-related changes in cortical bone, but were ineffective or much less efficient in this respect in trabecular bone (Rouffet et al., 1994; de Castro et al., 2010). In addition, local daily injection of PTHrP-107-139 for 5 days into normal adult mouse calvariae decreased bone resorption, and also reduced the number of osteoblasts to a lower extent at nanomolar concentrations, without significant change in osteoid area but with a trend to an increased mineralization (Cornish et al., 1997). Thus, it seems that in conditions of normal or high bone turnover in the experimental settings referred to above, the putative anabolic action of C-terminal PTHrP at least in some types of bone might be overshadowed by its concomitant effects on bone resorption. This contrasts with the anabolic efficacy of PTH, a well-characterized bone anabolic factor with proresorptive features, which was improved by simultaneous administration of antiresorptive agents in ovariectomized mice (Goltzman, 2008).
The probable anabolic action of PTHrP-107-139 as strongly suggested by our present data in a diabetic scenario was also supported by using BMSCs from control mice exposed to high glucose. Similar to what we observed in these cells isolated from diabetic mice, exposure to high glucose was shown to hamper osteogenesis (and to increase adipogenesis), and this was reversed by PTHrP-107-139. This peptide was effective even for short pulses, which is congruent with previous findings in other osteoblastic cell systems (Cornish et al., 1999; de Gortázar et al., 2006; Alonso et al., 2008). PTHrP-107-139 was also shown to reverse the deleterious effects of high glucose on osteoblastic maturation in more differentiated MC3T3-E1 cells. In addition, this peptide, in contrast to PTHrP-109-138, increased the expression of several osteoblast differentiation genes in these cells in normal glucose medium. The efficacy of PTHrP-107-139 to increase OPG mRNA expression in these cells, as observed here, is not surprising considering the various effects of this peptide in other osteoblastic cell preparations (Cornish et al., 1999; Valín et al., 2001; Alonso et al., 2008).
The present data also demonstrate that PTHrP-107-139 reversed the down-regulated PTH1R gene expression by high glucose in BMSCs and MC3T3-E1 cells in vitro, and also in vivo in the diabetic mouse tibiae. However, the efficacy of PTHrP-107-139 was unaltered by the presence of a PTH1R antagonist, indicating that this receptor is unlikely to mediate its osteoblastic actions. At the same time, either antagonizing PTH1R signalling (Lozano et al., 2009) or addition of a neutralizing antibody with C-terminal PTHrP specificity (as shown here) similarly mirrored the deleterious effects of high glucose on MC3T3-E1 cell differentiation. In addition, combined PTHrP-107-139 and PTHrP-1-36 showed the same efficacy as each single peptide in up-regulating OPG expression, and that of other osteoblastic genes (Esbrit et al., 2000; Guillén et al., 2002). These data lend credence to the hypothesis that a cross-talk in signal transduction pathways triggered by both C- and N-terminal domains of PTHrP occurs in osteoblastic cells (Valín et al., 2001; Hildreth et al., 2010). In this respect, previous findings underpin the putative role of mitogen-activated kinase activation as a key downstream mechanism to induce osteoblast differentiation by these PTHrP domains (Carpio et al., 2001; Chen et al., 2004; de Gortázar et al., 2006). Additional studies are needed to clarify this particular point in a diabetic scenario.
In conclusion, by using diabetic mice with low-turnover osteopaenia and an in vitro approach mimicking the diabetic condition, we showed for the first time that PTHrP-107-139 exerts true anabolic effects to promote bone formation, independent of its putative suppressive action on osteoclast function.
Acknowledgments
We thank F. Roncal, PhD. (Proteomics Unit, Centro Nacional de Biotecnología, Madrid, Spain) for human PTHrP-107-139 synthesis, and A.F. Stewart, MD and A. García-Ocaña, PhD. (Department of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA) for generously supplying PTHrP-1-36 and PTHrP-109-138. We are also indebted to M. Davis for proofreading the manuscript. This work was supported by grants from the Spanish Ministerio de Educación y Cultura (SAF2005-05254), Instituto de Salud Carlos III (PI050117, PI080922 and RETICEF RD06/0013/1002 and /1006) and Fundación de Investigación Médica Mutua Madrileña. DL, LF de C and AL-H are fellows of Fundación Conchita Rábago. SP-N was the recipient of a research contract from RETICEF RD06/0013/1002.
Glossary
Abbreviations
- µCT
microcomputed tomography
- ALP
alkaline phosphatase
- BMC
bone marrow cells
- BMD
bone mineral density
- DXA
dual-energy X-ray absorptiometry
- FABP
adipocyte fatty acid–binding protein
- NLS
nuclear localization signal
- OC
osteocalcin
- OPG
osteoprotegerin
- PPARγ2
peroxisome proliferator-activated receptor
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone-related protein
- PTH1R
parathyroid hormone receptor 1
- STZ
streptozotozin
- TNFRSF11A
receptor activator of nuclear factor-κβ ligand
- TRAP
tartrate-resistant acid phosphatase
- VEGF
vascular endothelial growth factor
Conflict of interest
None of the authors have any relationships with companies that may have a financial interest in the content of this manuscript or any other interest to disclose.
Supplementary material
Supporting Information: Teaching Materials; Figs 1–8 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4rd edition. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso V, de Gortázar AR, Ardura JA, Andrade-Zapata I, Alvarez-Arroyo MV, Esbrit P. Parathyroid hormone-related protein (107-139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2. J Cell Physiol. 2008;217:717–727. doi: 10.1002/jcp.21547. [DOI] [PubMed] [Google Scholar]
- Barbara A, Delannoy P, Denis BG, Marie PJ. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism. 2004;53:532–537. doi: 10.1016/j.metabol.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Bisello A, Horwitz MJ, Stewart AF. Parathyroid hormone-related protein: an essential physiological regulator of adult bone mass. Endocrinology. 2004;145:3551–3553. doi: 10.1210/en.2004-0509. [DOI] [PubMed] [Google Scholar]
- Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- Botolin S, Faugère M-C, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferators-activated receptor-γ2 expression in type I diabetic mice. Endocrinology. 2005;146:3622–3631. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio L, Gladu J, Goltzman D, Rabbani SA. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. Am J Physiol Endocrinol Metab. 2001;281:E489–E499. doi: 10.1152/ajpendo.2001.281.3.E489. [DOI] [PubMed] [Google Scholar]
- de Castro LF, Lozano D, Dapía S, Portal-Nuñez S, Caeiro JR, Gomez-Barrena E, et al. Role of the N- and C-terminal fragments of parathyroid hormone-related protein as putative therapies to improve bone regeneration under high glucocorticoid treatment. Tissue Eng Part A. 2010;16:1157–1168. doi: 10.1089/ten.TEA.2009.0355. [DOI] [PubMed] [Google Scholar]
- Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, et al. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem. 2004;279:29121–29129. doi: 10.1074/jbc.M313000200. [DOI] [PubMed] [Google Scholar]
- Chen H-L, Demiralp B, Schneider A, Koh AJ, Silve C, Wang C-Y, et al. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem. 2002;277:19374–19381. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- Connor JR, Dodds RA, James IE, Gowen M. Human osteoclast and giant cell differentiation: the apparent switch from nonspecific esterase to tartrate resistant acid phosphatase activity coincides with the in situ expression of osteopontin mRNA. J Histochem Cytochem. 1995;43:1193–1201. doi: 10.1177/43.12.8537635. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Nicholson GC, Reid IR. Parathyroid hormone-related protein-(107-139) inhibits bone resorption in vivo. Endocrinology. 1997;138:1299–1304. doi: 10.1210/endo.138.3.4990. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Lin C, Xiao C, Moseley JM, Reid IR. Stimulation of osteoblast proliferation by C-terminal fragments of parathyroid hormone-related protein. J Bone Miner Res. 1999;14:915–922. doi: 10.1359/jbmr.1999.14.6.915. [DOI] [PubMed] [Google Scholar]
- Cuthbertson RM, Kemp BE, Barden JA. Structure study of osteostatin PTHrP[Thr107](107-139) Biochim Biophys Acta. 1999;1432:64–72. doi: 10.1016/s0167-4838(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Dean T, Vilardaga JP, Potts JT, Jr, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbrit P, Alvarez-Arroyo MV, De Miguel F, Martín O, Martínez ME, Caramelo C. C-terminal parathyroid hormone-related protein increases vascular endothelial growth factor in human osteoblastic cells. J Am Soc Nephrol. 2000;11:1085–1092. doi: 10.1681/ASN.V1161085. [DOI] [PubMed] [Google Scholar]
- Fenton AJ, Kemp BE, Hammonds RG, Mitchelhill K, Moseley JM, Martin TJ, et al. A potent inhibitor of osteoclastic bone resorption within a highly conserved pentapeptide region of PTHrP (107-111) Endocrinology. 1991a;129:3424–3426. doi: 10.1210/endo-129-6-3424. [DOI] [PubMed] [Google Scholar]
- Fenton AJ, Kemp BE, Kent GN, Moseley JM, Zheng MH, Rowe DJ, et al. A carboxyl-terminal peptide from the parathyroid hormone-related protein inhibits bone resorption by osteoclasts. Endocrinology. 1991b;129:1762–1768. doi: 10.1210/endo-129-4-1762. [DOI] [PubMed] [Google Scholar]
- Goltzman D. Studies on the mechanisms of the skeletal anabolic action of endogenous and exogenous parathyroid hormone. Arch Biochem Biophys. 2008;473:218–224. doi: 10.1016/j.abb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- de Gortázar AR, Alonso V, Alvarez-Arroyo MV, Esbrit P. Transient exposure to PTHrP-107-139 exerts anabolic effects through vascular endothelial growth factor receptor 2 in human osteoblastic cells in vitro. Calcif Tissue Int. 2006;79:360–369. doi: 10.1007/s00223-006-0099-y. [DOI] [PubMed] [Google Scholar]
- Guillén C, Martínez P, de Gortázar AR, Martínez ME, Esbrit P. Both N- and C-terminal domains of parathyroid hormone-related protein increase interleukin-6 by NF-κB activation in osteoblastic cells. J Biol Chem. 2002;277:28109–28117. doi: 10.1074/jbc.M111013200. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone. 2007;40:1408–1414. doi: 10.1016/j.bone.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Hayman AR, Macary P, Lehner PJ, Cox TM. Tartrate-resistant acid phosphatase (Acp 5): identification in diverse human tissues and dendritic cells. J Histochem Cytochem. 2001;49:675–683. doi: 10.1177/002215540104900601. [DOI] [PubMed] [Google Scholar]
- Hildreth BE, Hildreth BE, 3rd, Werbeck JL, Thudi NK, Deng X, Rosol TJ, et al. PTHrP 1-141 and 1-86 increase in vitro bone formation. J Surg Res. 2010;16:e9–e17. doi: 10.1016/j.jss.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer L, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- Horwitz MJ, Tedesco MB, Gundberg C, Garcia-Ocaña A, Stewart AF. Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88:569–575. doi: 10.1210/jc.2002-021122. [DOI] [PubMed] [Google Scholar]
- Inzerillo A, Epstein S. Osteoporosis and diabetes mellitus. Rev Endocrine Metab Dis. 2004;5:261–268. doi: 10.1023/B:REMD.0000032415.83124.20. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, López-Luna P, Ortega A, Romero M, Gutiérrez-Tarrés MA, Arribas I, et al. The parathyroid hormone-related protein system and diabetic nephropathy outcome in streptozotocin-induced diabetes. Kidney Int. 2006;12:2171–2177. doi: 10.1038/sj.ki.5000195. [DOI] [PubMed] [Google Scholar]
- Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Fritton JC, Yakar S, Epstein S, Schaffler MB, Jepsen KJ, et al. Type 2 diabetic mice demonstrate slender long bones with increased fragility secondary to increased osteoclastogenesis. Bone. 2009;44:648–655. doi: 10.1016/j.bone.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Jun JH, Woo KM, Ryoo HM, Kim GS, Baek JH. Dexamethasone inhibits the formation of multinucleated osteoclasts via down-regulation of beta3 integrin expression. Arch Pharm Res. 2006;29:691–698. doi: 10.1007/BF02968254. [DOI] [PubMed] [Google Scholar]
- Legakis I, Mantouridis T. Positive correlation of PTH-related peptide with glucose in type 2 diabetes. Exp Diabetes Res. 2009;2009:291027. doi: 10.1155/2009/291027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D, de Castro LF, Dapía S, Andrade-Zapata I, Manzarbeitia F, Alvarez-Arroyo MV, et al. Role of parathyroid hormone-related protein in the decreased osteoblast function in diabetes-related osteopenia. Endocrinology. 2009;150:2027–2035. doi: 10.1210/en.2008-1108. [DOI] [PubMed] [Google Scholar]
- Lozano D, Manzano M, Doadrio JC, Salinas AJ, Vallet-Regí M, Gómez-Barrena E, et al. Osteostatin-loaded bioceramics stimulate osteoblastic growth and differentiation. Acta Biomater. 2010;6:797–803. doi: 10.1016/j.actbio.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144:346–352. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem. 2007;102:1343–1357. doi: 10.1002/jcb.21573. [DOI] [PubMed] [Google Scholar]
- Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest. 2005;115:2322–2324. doi: 10.1172/JCI26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao D, Su H, He B, Gao J, Xia Q, Zhu M, et al. Severe growth retardation and early lethality in mice lacking the nuclear localization sequence and C-terminus of PTH-related protein. Proc Natl Acad Sci U S A. 2008;105:20309–20311. doi: 10.1073/pnas.0805690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel F, Fiaschi-Taesch N, López-Talavera JC, Takane KK, Massfelder T, Helwig JJ, et al. The C-terminal region of PTHrP, in addition to the nuclear localization signal, is essential for the intracrine stimulation of proliferation in vascular smooth muscle cells. Endocrinology. 2001;142:4096–4105. doi: 10.1210/endo.142.9.8388. [DOI] [PubMed] [Google Scholar]
- Murrills RJ, Stein LS, Dempster DW. Lack of significant effect of carboxyl-terminal parathyroid hormone-related peptide fragments on isolated rat and chick osteoclasts. Calcif Tissue Int. 1995;57:47–51. doi: 10.1007/BF00298996. [DOI] [PubMed] [Google Scholar]
- Nuche-Berenguer B, Moreno P, Esbrit P, Dapía S, Caeiro JR, Cancelas J, et al. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009;84:4534–4561. doi: 10.1007/s00223-009-9220-3. [DOI] [PubMed] [Google Scholar]
- Nuche-Berenguer B, Moreno P, Portal-Nuñez S, Dapía S, Esbrit P, Villanueva-Peñacarrillo ML. Exendin-4 exerts osteogenic actions in insulin-resistant and type 2 diabetic states. Regul Pept. 2010;159:61–66. doi: 10.1016/j.regpep.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Okuda N, Takeda S, Shinomiya K, Muneta T, Itoh S, Noda M, et al. ED-71, a novel vitamin D analog, promotes bone formation and angiogenesis and inhibits bone resorption after bone marrow ablation. Bone. 2007;40:281–292. doi: 10.1016/j.bone.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Rouffet J, Coxam V, Gaumet N, Barlet JP. Preserved bone mass in ovariectomized rats treated with parathyroid-hormone-related peptide (1-34) and (107-111) fragments. Reprod Nutr Dev. 1994;34:473–481. doi: 10.1051/rnd:19940508. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD, Lynch MA, McKenzie JA, Tanouye KM, Nyman JS, et al. Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J Bone Miner Res. 2009;24:1618–1627. doi: 10.1359/JBMR.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AF, Cain RL, Burr DB, Jacob D, Turner CH, Hock JM. Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: a comparison of human parathyroid hormone 1-34, parathyroid hormone-related protein 1-36, and SDZ-parathyroid hormone 893. J Bone Miner Res. 2000;15:1517–1525. doi: 10.1359/jbmr.2000.15.8.1517. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugimoto C, Takizawa M, Ishizuka S, Kikuyama M, Togawa H, et al. Correlations between bone mineral density and circulating bone metabolic markers in diabetic patients. Diabetes Res Clin Pract. 2000;48:185–191. doi: 10.1016/s0168-8227(00)00119-4. [DOI] [PubMed] [Google Scholar]
- Toribio RE, Brown HA, Novince CM, Marlow B, Hernon K, Lanigan LG, et al. The midregion, nuclear localization sequence, and C terminus of PTHrP regulate skeletal development, hematopoiesis, and survival in mice. FASEB J. 2010;24:1947–1957. doi: 10.1096/fj.09-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valín A, García-Ocaña A, De Miguel F, Sarasa JL, Esbrit P. Antiproliferative effect of the C-terminal fragments of parathyroid hormone-related protein, PTHrP-(107-111) and (107-139), on osteoblastic osteosarcoma cells. J Cell Physiol. 1997;170:209–215. doi: 10.1002/(SICI)1097-4652(199702)170:2<209::AID-JCP13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Valín A, Guillén C, Esbrit P. C-terminal parathyroid hormone-related protein (PTHrP) (107-139) stimulates intracellular Ca2+ through a receptor different from the type 1 PTH/PTHrP receptor in osteoblastic osteosarcoma UMR 106 cells. Endocrinology. 2001;142:2752–2759. doi: 10.1210/endo.142.7.8276. [DOI] [PubMed] [Google Scholar]
- Wittrant Y, Gorin Y, Woodruff K, Horn D, Abboud HE, Mohan S, et al. High D(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008;42:1122–1130. doi: 10.1016/j.bone.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


