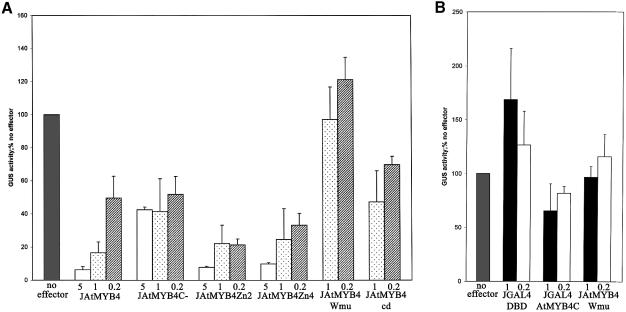
Fig. 9. (A) Analysis of the ability of AtMYB4 mutants to inhibit transcription from the C4H promoter in transfection assays. AtMYB4 repressedbasal expression of GUS from the C4H promoter between 20- and 40-fold depending on the concentration of effector supplied (5, 1 and 0.2 = 5, 1and 0.2 µg of effector plasmid, respectively). The repression exercised by AtMYB4 (JAtMYB4) was lifted by ∼50% by removal of the C-terminal domain of the protein (JAtMYB4C–). Mutation of either or both of the potential zinc finger domains had no effect on repression (JAtMYB4Zn2 and JAtMYB4Zn4). Mutation of the DNA binding domain of AtMYB4 (JAtMYB4Wmu) caused complete loss of all repression. Removal of the conserved domain (JAtMYB4cd) in the C-terminus of subgroup 4 family members reduced the ability of AtMYB4 to repress transcription, to a similar extent to complete removal of the C-terminus. Results are averages from at least four experiments. Error bars indicate standard deviations. (B) Analysis of the ability of the C-terminal domain of AtMYB4 to inhibit repression when linked to another DNA binding domain, using transfection assays. The GAL4 DNA binding domain slightly activated from the GAL4-90 promoter (JGAL4-DBD). Addition of the C-terminus of AtMYB4 to the GAL4 DNA binding domain (JGAL4AtMYB4C) reduced expression from the GAL4-90 promoter. This was not due to ‘squelching’ because the mutant of AtMYB4 with a defective DNA binding domain (JAtMYB4Wmu) had no effect on expression from the GAL4-90 promoter.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.