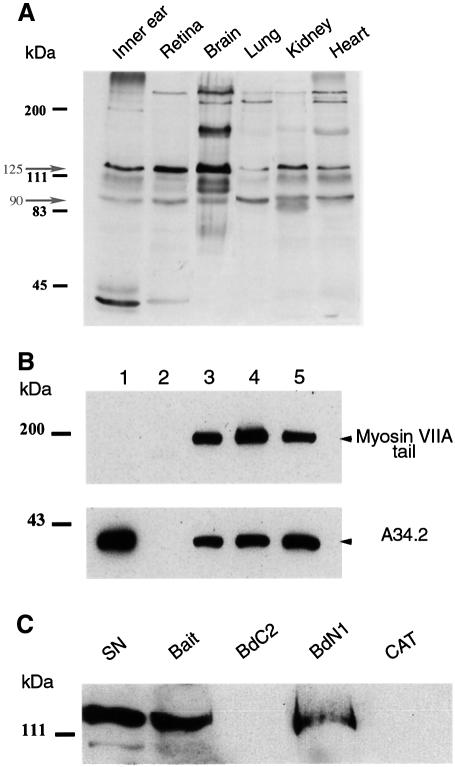
Fig. 2. Vezatin binds to the C-terminal FERM domain of myosin VIIA. (A) Expression of vezatin in adult mouse tissues. Total protein extracts (10 µg/lane) from six different tissues of 10-day-old mice were immunoblotted with the anti-mA34 antibody. Multiple vezatin isoforms are observed in all tissues. The higher bands may correspond to vezatin isoforms with large extracellular domains (see Figure 1). (B) Binding of vezatin to the myosin VIIA tail in co-transfected HEK293 cells. Extracts from co-transfected cells expressing both the myc-tagged A34.2 peptide and the myosin VIIA tail (lane 3) were used for co-immunoprecipitation experiments; the myosin VIIA tail and A34.2 peptide are co-immunoprecipitated with either the anti-myosin VIIA (lane 4) or the anti-myc (lane 5) antibody. When using extracts from HEK293 cells producing the myc-tagged A34.2 peptide alone (lane 1), no immunoprecipitate forms with the anti-myosin VIIA antibody (lane 2). (C) Binding of vezatin to the myosin VIIA C-terminal FERM domain. A standard amount of Caco-2 cell lysate containing endogenous vezatin (5% of which is shown in lane SN) was incubated with avidin resins coated with different biotinylated myosin VIIA peptides (bait, amino acids 1752–2215; BdC2, 1752–1931; BdN1, 1896–2215) or a biotinylated control protein, chloramphenicol acetyltransferase (CAT). Vezatin binds to either the bait peptide or the BdN1 fragment containing only the FERM domain, but not to BdC2 lacking the FERM domain or to CAT.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.