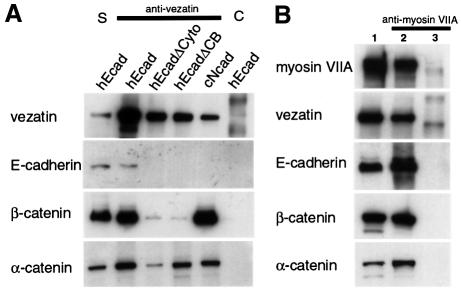
Fig. 6. Immunoprecipitation experiments in transfected cells expressing different forms of E-cadherin. (A) Extracts from L cells expressing the entire human E-cadherin (hEcad), human E-cadherin lacking the cytodomain (hEcadΔCyto) or the β-catenin binding domain (hEcadΔCB) and from S180 cells expressing the entire chicken N-cadherin (cNcad) were used. Vezatin, α-catenin and β-catenin are co-immunoprecipitated by an anti-vezatin antibody in cells transfected with either E- or N-cadherin cDNAs. In addition, vezatin and α-catenin are co-immunoprecipitated in cells producing the truncated E-cadherin variants (lanes hEcadΔCyto and hEcadΔCB), whereas only a very small fraction of β-catenin, compared with control (hEcad), is detected in the immunoprecipitate. The preimmune serum (lane C) was used as a negative control. S, soluble fraction. (B) In extracts from HEK293 cells expressing myosin VIIA tail (lane 2) the myosin VIIA tail co-immunoprecipitates with vezatin, E-cadherin, β-catenin and α-catenin, using an anti-myosin VIIA antibody. No immuno precipitation was observed with extracts from non-transfected cells (lane 3). Lane 1 contains the soluble fraction.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.