Abstract
PURPOSE
Young individuals with occlusive, proximal limb DVT who have acutely elevated plasma levels of factor VIII and D-dimer are at high risk for post-thrombotic syndrome (PTS) when treated with conventional anticoagulation alone. We sought to evaluate our experience with adjunctive percutaneous mechanical/pharmacomechanical thrombolysis (PMT/PPMT) in such patients.
PATIENTS AND METHODS
Among 95 children 11 to 21 years of age enrolled in a prospective cohort of venous thromboembolism between March 1, 2006 and November 1, 2009, 16 met eligibility criteria and underwent PMT/PPMT, typically with adjunctive catheter-directed thrombolytic infusion (CDTI) of tissue-type plasminogen activator given post-procedure.
RESULTS
Median age was 16 years (range: 11–19 years). Thirteen cases (81%) involved lower limbs. Underlying stenotic lesions were disclosed in 53%, with endovascular stents deployed in all cases of May-Thurner. There were no peri-procedural major bleeding events and one symptomatic pulmonary embolism. Technical success rate was 94%. Early (<30 days) locally recurrent DVT developed in 40%, of which 83% were successfully re-lysed. Late recurrent DVT rate (median follow-up duration: 14 months [range: 1–42 months]) was 27%. Cumulative incidence of physically and functionally significant PTS at 1–2 years was 13%.
CONCLUSION
This experience provides prospective evidence that PMT/PPMT with adjunctive CDTI can be used safely and effectively in adolescents with DVT at high risk for PTS.
Introduction
Current consensus-based recommendations for antithrombotic therapy of deep venous thrombosis (DVT) in children suggest that thrombolytic interventions be reserved for patients with life- or limb-threatening events [1]. However, a risk of post-thrombotic syndrome (PTS) of nearly 80% was recently determined in a small cohort study evaluating a subgroup of young patients with DVT—defined by completely veno-occlusive proximal lower limb DVT in association with acutely elevated plasma levels of factor VIII and D-dimer—managed by conventional anticoagulation according to the present standard of care [2]. This study further observed that when thrombolysis (typically consisting of systemic tissue-type plasminogen activator [tPA] by low-dose continuous intravenous infusion, with salvage percuatenous mechanical or pharmacomechanical thrombolytic [PMT/PPMT] intervention for persistent occlusion) was employed prior to conventional anticoagulation, the risk of PTS was significantly reduced (OR=0.02, 95% confidence interval=<0.001–0.48), at 22%. This PTS rate is similar to the historical frequency of PTS recently defined in largely unselected children with a history of upper or lower extremity DVT by systematic review [3].
Present recommendations for antithrombotic management of DVT in adults suggest that thrombolysis be considered in young patients with a long anticipated lifespan who have a low bleeding risk and extensive and/or occlusive thrombus of recent symptomatic onset [4]. Since most children with PTS are anticipated to endure the associated symptoms and signs for a lifetime that spans many decades, it is imperative that risk-stratified antithrombotic therapeutic strategies be developed and prospectively studied in the pediatric population, with PTS risk reduction as a key aim. Principal among these strategies is the rapid restoration of venous patency via thrombolysis. To date, the few studies of thrombolysis in pediatric venous thromboembolism (VTE) have focused upon modalities of either systemic or catheter-directed thrombolytic infusion (CDTI), including tPA most recently and urokinase in the recent past [2,5,6]. However, a recent survey identified PMT/PPMT as the most commonly preferred approach to thrombolysis in pediatric DVT among physician members of the American Society of Pediatric Hematology/Oncology [7], even in the absence of any published experience apart from a few small retrospective case series. Accordingly, we sought to evaluate our institutional prospective cohort study experience with PMT/PPMT in adolescents with acute proximal limb DVT who were judged to be at high risk for PTS due to the combined presence of complete veno-occlusion and adverse prognostic biomarkers.
Methods
Subjects
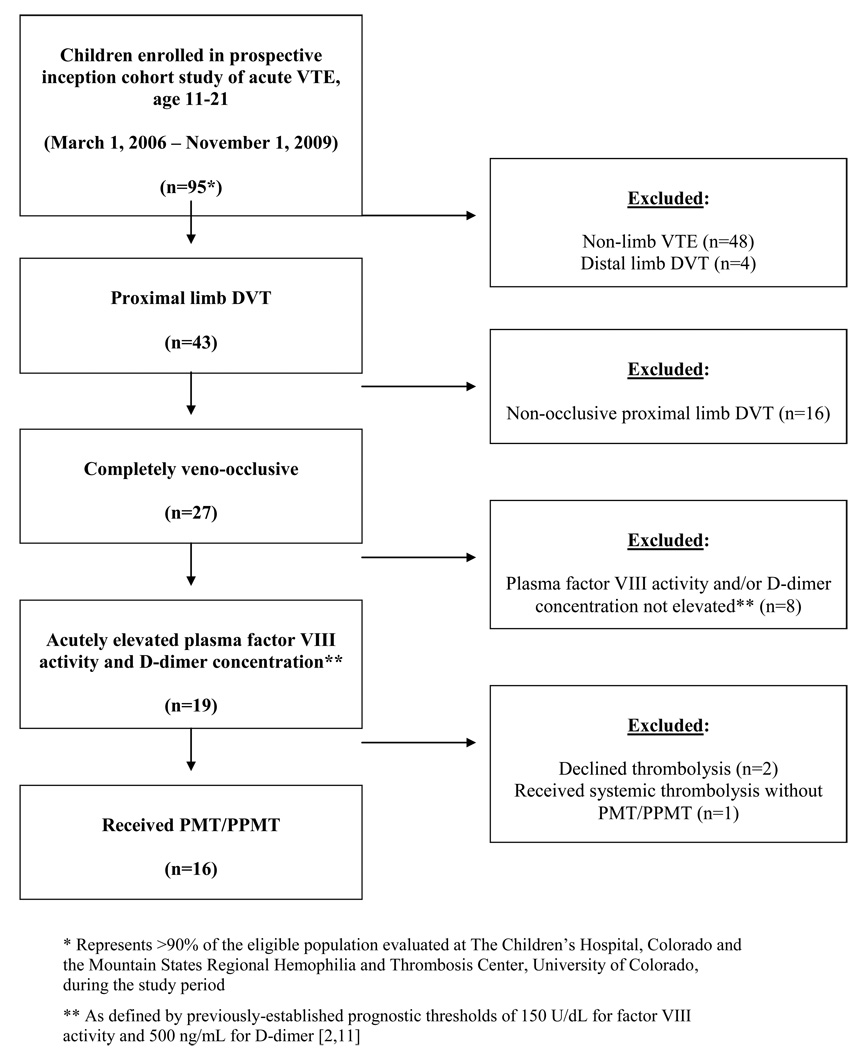
Consecutive children evaluated for VTE at The Children’s Hospital, Colorado and the Mountain States Regional Hemophilia and Thrombosis Center at the University of Colorado Denver were approached for enrollment in an institution-based prospective inception cohort study of pediatric VTE between March 1, 2006 and November 1, 2009 (Colorado Multiple Institutional Review Board approval #05–0339). Informed consent was provided in accordance with the Declaration of Helsinki. Study inclusion criteria for the present analysis consisted of: (1) Age ≥11 and ≤21 years at presentation; (2) symptomatic, completely veno-occlusive DVT of a proximal limb, objectively confirmed via appropriate radiologic imaging studies (compression ultrasound with Doppler, computed tomography with venography [CTV], magnetic resonance imaging with venography [MRV], conventional venography); (3) plasma factor VIII activity and D-dimer concentration above prognostic thresholds (150 U/dL and 0.5 µg/mL, respectively) upon initial testing within two weeks following DVT confirmation; (4) PMT/PPMT via an interventional radiologic approach; (5) prospective follow-up in the Pediatric Thrombosis Program at the University of Colorado Denver and The Children’s Hospital, Colorado; (6) signed informed consent/assent for study participation. The only exclusion beyond these eligibility criteria was a lag to PMT/PPMT of ≥ 60 days from time of symptom onset. Figure 1 provides a flow chart of study population eligibility.
Figure 1.
Flow chart of subject selection from the cohort study population, based upon clinical and laboratory characteristics of interest for heightened a priori risk of PTS.
PMT/PPMT regimens
Patients were carefully evaluated for contraindications to local thrombolysis (Figure 2); no children required exclusion for contraindications. In addition to informed research consent for cohort study participation, informed procedural consent was obtained in all cases. A clinical protocol was employed for each component of the PMT/PPMT intervention (Figure 3), which permitted variability in the number of modalities utilized, based upon clinical discretion. In brief, baseline venography was performed to establish anatomy, thrombus burden, and degree of occlusion. Using realtime ultrasound guidance, a 21-gauge needle was introduced into the popliteal vein for lower limb DVT or the basilic veins for upper limb DVT. In the setting of thrombus within one of these two venous systems, puncture was made peripheral to the thrombosed vein and an attempt at passing a wire through the thrombus was made. Via standard wire and catheter exchanges, a hydrophilic wire was advanced through the thrombus to its proximal (central) terminus and a central venogram was obtained. An appropriately-sized sheath was then placed over the guidewire.
Figure 2.
Observed relative contraindications to local thrombolytic invervention. These relative contraindications were adapted from absolute and relative contraindications to systemic thrombolysis. Contraindications were considered relative based upon the possibility of systemic exposure to thrombolytic agent in small amounts during PMT, or in large amounts during CDTI once patency achieved. No patients were excluded for contraindications.
Figure 3.
Summary of clinical protocol for PMT/PPMT.
One of three mechanical thrombectomy devices (AngioJet® [MEDRAD Interventional/Possis, Inc., Warrendale, PA, USA]; Amplatz ClotBuster® Thrombectomy Device [ev3 Inc., Plymouth, MN, USA]; or Trellis® [Bacchus Vascular, Inc., Santa Clara, CA, USA]), ranging in caliber from 5–8 F, was advanced through the sheath. Mechanical thrombectomy was performed according to manufacturer specification, in serial passes as necessary/possible to achieve maximal patency and minimal residual clot burden. When local tPA was administered via the device during MT (i.e.: Trellis®; some cases of ClotBuster® or Angiojet®), this was characterized as PMT. At the discretion of the operator (or in all cases of May-Thurner anomaly), additional percutaneous transluminal angioplasty was performed and one or more 12–16 mm stents (WALLSTENT®, Boston Scientific, Natick, MA, USA) were deployed at sites of residual stenosis. If thrombus was noted to extend peripheral to the popliteal vein (i.e. including calf veins), no attempt was made to lyse this portion of the thrombus. No duplicate venous systems were identified during this study.
In most cases, catheter-directed thrombolytic infusion (CDTI) was administered post-procedure directly into residual thrombus, for a period of 12–24 hours. CDTI uniformly employed tPA at 0.5–1 mg/hr via an infusion catheter placed in the sheath and directed at the site of PMT/PPMT or residual thrombus. During CDTI, unfractionated heparin was given at 10 U/kg/hr via the sheath (except in cases in which a diagnosis of severe thrombophilia [e.g., homozygous protein S deficiency] or thrombotic storm [8] was made, wherein more aggressive antithrombotic therapy was maintained [Table 3]).
Table 3.
Outcomes in the study population.*
| Case # |
Acute locally recurrent DVT within 7d of procedure,† and status on repeat procedure (re-lysed vs. refractory) |
Follow-up duration at last clinic visit (mo) |
Non- acute recurrent VTE, and type (local vs. distant) |
Time from procedure to dx of non-acute recurrent VTE (mo) |
Any PTS at 1–2 y |
Physically and functionally significant PTS at 1–2 y |
Basic CEAP findings |
Wong- Baker chronic pain findings |
|---|---|---|---|---|---|---|---|---|
| 1 | None | 12 | Yes (distant) |
4.5 | Yes | n/a | C | n/a (pre- morbid chronic pain) |
| 2 | None | 42 | None | n/a | Yes | No | None | AA, ADL |
| 3 | Yes (re-lysed) |
36 | Yes (local) |
0.5 | Yes | No | None | AA |
| 4 | Yes (re-lysed) |
27 | Yes (local) |
14 | Yes | Yes | E | AA, ADL |
| 5 | None | 30 | None | n/a | Yes | No | None | AA |
| 6 | None | 24 | None | n/a | No | No | None | None |
| 7 | Yes (re-lysed) |
2 | None | n/a | n/a | n/a | n/a | n/a |
| 8 | Yes (re-lysed§) |
14 | Yes (distant) |
3–12 mo (asymptomatic) |
No¶ | No | None | None |
| 9 | None | 26 | Yes (local) |
1–3 mo (asymptomatic) |
Yes | No | E | None |
| 10 | None | 14 | None | n/a | Yes | No | E | None |
| 11 | None | 18 | Yes (local) |
2 mo | Yes | No | None | AA, rest |
| 12 | None | 6 | None | n/a | n/a | n/a | n/a | n/a |
| 13 | None | 10 | Yes (local) |
9 mo | n/a | n/a | n/a | n/a |
| 14 | None | 3 | None | n/a | n/a | n/a | n/a | n/a |
| 15 | Yes (re-lysed§) |
1 | None | n/a | n/a | n/a | n/a | n/a |
| 16 | None | 6 | None | n/a | n/a | n/a | n/a | n/a |
Abbreviations: DVT=deep venous thombosis; PE=pulmonary embolism; PTS=post-thrombotic syndrome; CEAP= Basic Clinical-Etiologic-Anatomic-Pathophysiologic (American Venous Forum) physical exam component of the Manco-Johnson pediatric PTS instrument; W-B: Wong-Baker “faces” chronic pain
There were no major bleeding events, and one symptomatic pulmonary embolism, within 7 days of procedure.
Includes isolated stent thrombosis.
Reliability of assessment of basic CEAP component on contralateral difference in limb circumference may be limited due to bilaterality of DVT at presentation.
Recurred twice in these subjects with multiple-antibody APS with thrombotic storm. Patency was eventually maintained post-MT/PMT in each patient only after multi-modal immunomodulatory therapy had been instituted and potent direct thrombin inhibition had resulted in marked decrease in D-dimer.
Complete blood count, prothrombin time, activated partial thromboplastin time, fibrinogen activity (Clauss method), plasminogen activity, and D-dimer concentration were measured every 12 hours during, and for 24 hours after, the thrombolytic regimen. In the absence of bleeding concerns, therapeutic anticoagulation was transitioned to low molecular weight heparin (LMWH) 24-hours post-procedure, monitored by anti-Xa activity (goal: 0.5–1.0 anti-Xa U/mL). Anticoagulation was maintained with LMWH or warfarin (goal INR: 2.0–3.0) for a minimum total duration of six months, and not less than three months from the time of any stent placement. Duration of anticoagulation was guided by American College of Chest Physicians consensus-based recommendations [1].
Data collection
Systematic, prospective collection of clinically-derived data included: patient demographics; timing of symptom onset; thrombus site, extent and degree of occlusion; clinical risk factors for DVT; thrombophilia findings; presence of May-Thurner anomaly or Paget-Schroetter syndrome; interventions and medical regimens employed, and timing thereof; peri-procedural (≤7 days) major bleeding complications; peri-procedural pulmonary embolism (PE); technical success (end-of-intervention); rethrombosis (i.e., locally recurrent DVT); all recurrent VTE (all sites); and PTS. Major hemorrhage was defined as bleeding involving or resulting in any one of the following: central nervous system; retroperitoneum; decline in hemoglobin of ≥2 g/dL in a 24 hour period; need for invasive procedure to achieve hemostasis; death. In accordance with previously established reporting standards, technical success was characterized by grade II/III (>50%) lysis, early locally recurrent DVT was defined as that which was objectively confirmed within 30 days post-intervention, and late recurrent DVT as greater than 30 days post-procedure [9].
Based upon institutionally-defined standard/best clinical care, all children underwent comprehensive thrombophilia testing as adapted from International Society of Thrombosis and Haemostasis recommendations [10] as previously described [11], in order to define risk factors for DVT onset and/or its recurrence. Severe deficiencies of coagulation regulatory proteins were defined as antithrombin <30 U/dL and protein C or protein S <20 U/dL), while mild deficiencies were defined as antithrombin 30–60 U/dL and protein C or S 20–40 U/dL. These deficiencies were further distinguished as inherited versus acquired based upon persistence versus non-persistence of the abnormality upon follow-up assessment 12 weeks or more from the time of initial testing, with supportive evidence provided via testing of family members.
Radiologic imaging surveillance was performed in order to exclude thrombus progression/recurrence on therapy as well as to define the extent of chronic thrombus burden of any residual thrombus. Specifically, imaging was repeated at end of PMT intervention, 1–4 weeks, 3–6 months, and (if persistent) at 1 year. Additionally, children were evaluated for PTS using the Manco-Johnson instrument at 3–6 months, 1 year, and annually thereafter in long-term follow-up. As reported previously [12], the Manco-Johnson instrument consists of a physical exam component (basic CEAP [13]) as well as a functional component (assessment of chronic pain with age-appropriate aerobic activities, activities of daily living, and at rest, using the Wong-Baker scale [14]). Validation data have been published for the use of the Manco-Johnson instrument in pediatric PTS assessment following DVT of the lower limbs [2], and have likewise been recently presented for non-catheter-related DVT of the upper limbs [15].
Statistical methods
Descriptive statistics were employed to define distributions of continuous data and frequencies/proportions of categorical data. Ninety-five percent confidence intervals (95% CI) were calculated using the Wald method. Cumulative incidence of PTS (expressed as a proportion) was calculated based upon findings at 1–2 years post-diagnosis; findings from the 2-year evaluation were used for this analysis, when available.
In the hypothesis tests, distributions and proportions were compared between groups of interest via Mann-Whitney U and chi-square testing, respectively, with Fisher’s exact test used for circumstances in which frequency values were ≤5 in a 2-by-2 table. An alpha of 0.05 was considered statistically significant. All hypothesis tests were two-sided, and were performed using SAS 9.1 statistical software (SAS Institute, Cary, NC).
Results
Study population characteristics
Among 91 children aged 11–21 (inclusive) with VTE who enrolled in an institution-based prospective cohort study between March 1, 2006 and November 1, 2009, 19 had completely-occlusive proximal limb DVT in association with acute elevation of FVIII and D-dimer (Figure 1). Of these, 16 (18% of enrolled subjects with VTE) elected to undergo PMT/PPMT as part of clinical care within 60 days of symptom onset, after consideration of risks and benefits of treatment options. These 16 adolescents comprise the study population for the present analysis. Demographics, baseline patient and thrombus characteristics, and DVT risk factors in the study population are presented in Table 1.
Table 1.
Demographics, baseline characteristics, and predisposing conditions of the study population.
| Case # |
Age (y) |
Gender | DVT Site(s) |
PE at presen- tation |
Lag time from sx onset to dx (d) |
Lag time from dx to thrombo- lytic rx (d) |
Clinical triggers |
Acute thrombo- philia findings |
Vascular anomaly |
Other diagnoses |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | F | L iliac, fem, pop |
No | 1 | 45 | Immobility | FVIII, LA, FVL het, PS (mild, acquired) |
May- Thurner |
Juvenile rheumatoid Arthritis |
| 2 | 17 | F | L iliac, fem, pop |
No | 3 | 0 | OCP Trauma |
FVIII, LA | May- Thurner |
APS |
| 3 | 16 | M | R SC, ax, basilic |
No | 10 | 0 | None | LA, beta-2- gp-I |
Paget- Schroetter |
APS |
| 4 | 17 | F | L iliac, Fem |
No | 7 | 26 | OCP Trauma |
n/a | May- Thurner |
APS |
| 5 | 16 | F | IVC, R iliac, fem, pop |
No | 3 | 0 | OCP EBV infection |
FVL het FVIII |
None | None |
| 6 | 15 | M | R iliac, fem |
No | 30 | 0 | Obesity | FVL het FVIII |
None | APS Trisomy 21 |
| 7 | 17 | M | R SC | No | 9 | 1 | Chronic effort (baseball pitcher) |
n/a | Paget- Schroetter |
None |
| 8 | 11 | M | R iliac, L iliac, IVC |
Yes | 7 | 4 | Trauma | FVIII, AT (mild, acquired), FVL het, LA, Lp(a) |
May- Thurner |
APS with thrombotic storm |
| 9 | 15 | M | L iliac, Fem |
No | 1 | 2 | Trauma | LA, Beta-2- GP1, ACA |
None | APS |
| 10 | 17 | F | R fem, pop, IVC |
Yes | 5 | 2 | Obesity, OCP |
FVIII, FVL het, LA |
None | None |
| 11 | 19 | M | L fem, pop |
No | 6 | 8 | ALL L-asp |
AT (mild, acquired), PS (mild, acquired), FVIII |
None | None |
| 12 | 17 | F | L iliac, fem, pop |
Yes | 6 | 12 | OCP | FVL het, PC (mild, inherited), FVIII |
None | None |
| 13 | 11 | F | L fem | Yes | 6 | 8 | None | PS (sev, inherited) LA |
May- Thurner |
None |
| 14 | 16 | M | IVC, L iliac, B fem |
No | 4 | 6 | None | LA, FVL/ PT 20210 dual het |
None | None |
| 15 | 18 | F | L iliac, Fem, pop |
No | 1 | 1 | Immobility | FVIII, LA, FVL het, PS (mild, acquired) |
May- Thurner |
APS |
| 16 | 14 | M | L SC, ax | Yes | 4 | 48 | None | FVIII, LA, AT (mild, acquired), PC (mild, acquired) |
n/a (obstruct- ted by thrombus) |
None |
Abbreviations: DVT=deep venous thrombosis; PE=pulmonary embolism; dx= diagnosis; rx=treatment; F=female; M=male; fem= femoral; pop=popliteal; IVC=inferior vena cava; SC=subclavian; BC=brachiocephalic; ax=axillary; SVC=superior vena cava; L= left; R=right; B=bilateral; OCP=oral contraceptive pill (containing estrogen); FVIII=elevated factor VIII activity; LA=lupus anticoagulant; PS=protein S deficiency; sev=severe; FVL het=heterozygous factor V Leiden polymorphism; n/a=not available or not applicable; EBV=Epstein-Barr virus; ALL-acute lymphoblastic leukemia; L-asp: L-asparaginase; APS=antiphospholipid antibody syndrome
Median age was 16 years (range: 11–19 years). Thirteen cases (81%) were lower limb DVT, nearly all of which involved the iliofemoral circulation. Four lower limb DVT cases (31%) involved the inferior vena cava (IVC). Underlying stenotic venous anomalies (specifically, May-Thurner anomaly or Paget-Schroetter syndrome) were definitively diagnosed in eight (53%) of 15 evaluable subjects (in subject #16, the thrombus was too organized to allow passage of a guidewire, and definitive diagnosis of Paget-Schroetter syndrome was therefore not possible due to subclavian involvement with residual occlusive thrombus). Endovascular stents were deployed in all cases of May-Thurner anomaly.
Treatment
Antithrombotic treatments, including thrombolytic interventions, are shown in Table 2 Five patients received low-dose systemic tPA (0.03–0.06 mg/kg/hr) by continuous intravenous infusion for 48–96 hrs prior to PMT/PPMT; in these cases, PMT/PPMT was initiated due to persistent veno-occlusion. PMT/PPMT was successfully conducted in 15 cases; CDTI was employed adjunctively in 11 (73%) of these. Retrievable IVC filters were placed in 6 subjects (38%). Median number of procedure days for PMT +/− CDTI was 2 (range: 1–4).
Table 2.
Thrombolytic modalities, anticoagulant therapies, and additional therapeutic measures employed in the study population.
| Case # |
PMT/ PPMT device* |
PTA used during procedure |
CDTI given post- procedure |
Stent deploy- ment (and site) |
Retrievable IVC filter placement (and successful retrieval?) |
Total # proce- dure days† |
Post-lytic inter- vention AC regimen¶ |
Extended AC Regimen |
Dura- tion of AC (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ClotBuster (PMT) |
Yes | No | Iliac | Yes (no) |
3 | LMWH | LMWH x 6 mo, then warfarin |
11+ |
| 2 | ClotBuster (PMT) |
Yes | Yes | Iliac | Yes (yes) |
2 | LMWH | Warfarin | 22 |
| 3 | AngioJet (PMT) |
Yes | Yes | None | No | 3§ | LMWH | Warfarin | 12 |
| 4 | AngioJet (PMT) |
Yes | Yes | Iliac | No | 2 | LMWH | Warfarin | 27+ |
| 5 | AngioJet (PMT) |
No | Yes | None | Yes yes) |
2 | LMWH | Warfarin | 3 |
| 6 | AngioJet (PMT) |
Yes | No | Iliac | Yes (yes) |
1 | LMWH | Warfarin | 18+ |
| 7 | AngioJet PPMT) |
Yes | No | None | No | 1§ | LMWH | Warfarin | 2+ |
| 8 | ClotBuster (PPMT) |
Yes | Yes | Iliac | Yes (no) |
4 | IV DTI | Fonda- parinux x 1 mo, then warfarin |
17 |
| 9 | ClotBuster (PPMT) |
Yes | Yes | None | No | 2 | LMWH | Warfarin | 26+ |
| 10 | Trellis (PPMT) |
Yes | Yes | None | Yes (yes) |
2 | LMWH | Warfarin | 14 |
| 11 | ClotBuster (PMT) |
No | Yes | None | No | 2 | LMWH | LMWH | 6 |
| 12 | ClotBuster (PMT) |
No | Yes | None | No | 2 | LMWH | Warfarin | 3+ |
| 13 | ClotBuster (PMT) |
No | Yes | Iliac | No | 3 | LMWH | Warfarin | 4+ |
| 14 | ClotBuster (PPMT) |
No | No | None | No | 2 | LMWH | Warfarin | 3+ |
| 15 | ClotBuster (PMT) |
No | Yes | Iliac | No | 2 | IV DTI | Fonda- parinux |
1+ |
| 16 | Failed (unable to insert guidewire into thrombus) |
No | No | None | No | 1 | LMWH | Warfarin | 2+ |
Abbreviations: AC=anticoagulant; tPA=tissue-type plasminogen activator; LD CIVI=; PMT=percutaneous mechanical thromoblysis (see Methods); PPMT=percutaneous pharmacomechanical thrombolysis (see Methods); CDTI: catheter-directed thrombolytic infusion (using tPA; see Methods); UFH: unfractionated heparin; LMWH: low molecular weight heparin; PTA=percutaneous transluminal balloon angioplasty; IV DTI=intravenous direct thrombin inhibitor
Systemic tPA given by low-dose (0.03-0.06 mg/kg/hr) continuous intravenous infusion prior to PMT/PPMT in cases #1–3, 6.
During initial hospitalization and within 10d post discharge
Immediate pre-procedural anticoagulation consisted of therapeutic UFH (goal anti-Xa activity = 0.3–0.7 U/mL) in all cases. UFH administration was suspended during PMT/PPMT procedure, except in cases #13 and 15, where UFH was maintained at subtherapeutic dosing of 10–12 U/kg/hr. UFH was maintained at 10 U/kg/h during CDTI, where employed.
Does not include partial 1st rib resection, performed in both cases of Paget-Schroetter syndrome.
Outcomes
Safety and efficacy outcomes are summarized in Table 3. With regard to safety, there were no peri-procedural major bleeding events and one symptomatic pulmonary embolism. As for efficacy, technical success (grade II/III lysis) was achieved in 94% (95% confidence interval [CI]: 70->99%) of cases. There were five acute local recurrences within one week (all of which were successfully re-lysed), one sub-acute, and four late local recurrences. Late local recurrences were restricted in all cases to patients with severe hypercoagulability, with or without underlying stenotic venous lesions, as follows: asparaginase-induced multiple thrombophilias (n=1); severe protein S deficiency with May-Thurner anomaly (n=1); antiphospholipid antibody syndrome (APS, n=1); and APS with May-Thurner anomaly (n=1). Using Society of Interventional Radiology outcomes reporting criteria [9; see also Methods], overall early (<30 days) and late (>30 days; median follow-up duration: 14 months [range: 1–42 months]) rethrombosis rates were therefore 40% (95% CI: 20–64%) and 27% (95% CI: 10–52%), respectively. Cumulative incidence of any PTS (physical findings on the basic CEAP component or chronic pain findings on the Wong-Baker component of the Manco-Johnson instrument) at 1–2 years was 80% (95% CI: 48–95%), while that of. both physically and functionally significant PTS (abnormalities on both components of the instrument) was 13% (95% CI: 0.1–49%).
Prognostic factors for PTS
The results of univariate analyses for prognostic factors with regard to therapeutic efficacy are shown in Table 4. While no statistically significant associations were detected (and while sample size per group was small), a tendency toward increased risk of physically and functionally significant PTS was observed among patients in whom: lag time from symptom onset to lytic intervention exceeded ten days; stenotic venous anomalies were disclosed (all of which were successfully stented); and PMT (rather than PPMT) was used. The latter observation was accompanied by a tendency toward increased rates of locally recurrent DVT for PMT in comparison to PPMT.
Table 4.
Investigation of putative prognostic factors for development of PTS in the study population.*
| Lag time | Stenotic venous lesion† |
APS | PPMT versus PMT† |
Adjunctive CDTI† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 10 d | > 10 d | Yes | No | Yes | No | PPMT | PMT | Yes | No | |
|
N (% of study population) |
8 (50%) | 8 (50%) | 8 (53%) | 7 (47%) | 7 (44%) | 9 (56%) | 5 (37%) | 10 (63%) | 11 (73%) | 4 (27%) |
|
Median age (range) (y) |
17 (15–18) |
16 (11–19) |
17 (11–18) |
16 (14–19) |
16 (11–18) |
16 (11–19) |
16 (11–17) |
17 (11–19) |
17 (11–19) |
16 (15–17) |
|
Gender (female n [group %]) |
4 (50%) | 4 (50%) | 5 (63%) | 3 (43%) | 3 (43%) | 5 (56%) | 1 (20%) | 7 (70%) | 7 (64%) | 1 (25%) |
|
Technical success (group %)¶ |
100% | 88% | 100% | 100% | 100% | 89% | 100% | 100% | 100% | 100% |
|
Rethrombosis (group %)§ |
12% | 12% | 25% | 0% | 29% | 11% | 0% | 20% | 18% | 0% |
|
Any PTS (group %) [ n evaluable] |
80% [n=5] |
50% [n=4] |
80% [n=5] |
50% [n=4] |
50% [n=6] |
100% [n=3] |
33% [n=3] |
83% [n=6] |
71% [n=7] |
50% [n=2] |
|
Physically and functionally significant PTS (group %) [ n evaluable] |
0% [n=5] |
33% [n=3] |
25% [n=4] |
0% [n=4] |
17% [n=6] |
0% [n=2] |
0% [n=3] |
20% [n=5] |
14% [n=7] |
0% [n=1] |
Abbreviations: PTS=post-thrombotic syndrome; APS=antiphospholipid antibody syndrome; PMT=percutaneous mechanical thromoblysis; PPMT=percutaneous pharmacomechanical thrombolysis; CDTI=catheter-directed thrombolytic infusion (using tPA); n=number of patients.
No statistically significant differences in intergroup distributions or proportions were detected. Note, however, that group same sizes were small.
Subject #16 (in whom acute patency was not achieved) is not represented in the venous anomaly, PMT-versus-PPMT, or CDTI strata given that definitive diagnosis and lytic interventions were not feasible.
As defined by grade II/III lysis (see also Methods).
Consists of early + late rethrombosis (see also Methods).
Discussion
The present findings provide prospective evidence that PMT/PPMT with/without adjunctive CDTI can be used safely in children, targeting a subgroup of adolescents with DVT known to be at high a priori risk for PTS. Peri-procedural symptomatic pulmonary embolism occurred in only one patient and major bleeding was absent among 16 consecutively enrolled cases in the cohort study. Despite acute locally recurrent DVT in 40%, 83% of these were successfully re-lysed. While late recurrent DVT occurred in 27% overall, all were in the setting of severe hypercoagulable states. Although signs and/or symptoms of PTS were still observed in some patients even when PMT/PPMT was performed within 10 days of symptom onset, the rate of functionally significant PTS occurred in only 13% of these high-risk patients. These findings identify PMT/PPMT with adjunctive CDTI as worthy of further prospective study as an effective and potentially safer (vis-à-vis systemic bleeding risk) alternative to systemic thrombolysis in children with occlusive proximal limb DVT who have adverse prognostic biomarkers at acute presentation.
Several additional observations in this study are important, including use of retrievable IVC filters and endovascular stents. Outcomes for the use of retrievable IVC filters in children have rarely been reported previously. The present finding of non-retrievability in two of six cases (33%) in which retrievable IVC filters were placed is consistent with a similar observation in one of six cases in the single prospective pediatric series of IVC filter use published to date [16]. In our series, non-retrievability was associated with accumulation of organized thrombus at the site where the filter legs apposed the IVC wall. In one case, there was a prolonged interval to filter retrieval (three months from time of placement). In the other, retrieval was within a conventional timeframe (six weeks from placement), but the patient’s course was complicated by prolonged, severe coagulation activation as multimodal immunosuppressive and antithrombotic therapies were being escalated for the treatment of thrombotic storm in APS. These cases highlight the importance of careful consideration of risks and benefits of retrievable IVC filters in children, and the need for collaborative studies to provide more extensive data on this issue in the context of a rare disease.
Our preliminary finding of a low risk of recurrent VTE following iliac vein stenting for May-Thurner anomaly in children without sever underlyinig thrombophilia is supported by the two prior series (both retrospective) in this area [17,18]. None of the patients ≤ 21 years of age in these reports suffered recurrent VTE, at median follow-up durations of 11 and 18 months. Like our study, these prior series involved PMT/PPMT with/without CDTI prior to stent placement. Our prospective series involved six cases of May-Thurner anomaly. Among the five adolescents with May-Thurner anomaly in whom PMT/PPMT was performed within six weeks of symptom onset, with a median follow-up duration of 10 months, locally recurrent DVT developed in two subjects, both with severe underlying thrombophilia. In another case, recurrent VTE consisted of a distant event (subclavian DVT at the site of peripherally-inserted central catheter) unrelated to May-Thurner anomaly. Taken together, these experiences suggest that, in a disorder for which risk of recurrent VTE historically appears to be high, interventional thrombolysis with iliac vein stent placement may provide an effective means of secondary VTE prevention. However, our sample size with May-Thurner anomaly is small; as with the case of retrievable IVC filters, larger efforts are needed to adequately investigate this issue.
A recent systematic review of the literature revealed a weighted mean frequency of PTS in children of nearly 25%, albeit with considerable variability across eligible studies [3]. Pediatric findings with the use of a standardized PTS scoring system (adapted from Villalta scale) were first published in 2003 in a retrospective analysis by the Childhood Thrombophilia Program at the Hospital for Sick Children [19]; this study reported a prevalence of PTS of 63%. Prospective evidence using a pediatric PTS outcome measure, the Manco-Johnson instrument (for which validation data were subsequently published [2]), has indicated a cumulative incidence of PTS (as defined by physical or functional findings) of 33% at 1–2 years among unselected children [11]. In addition, a cumulative incidence of 77% was observed at approximately 2 years using the Manco-Johnson instrument in a small cohort of children with completely-occlusive lower limb DVT treated with standard anticoagulation in whom plasma levels of factor VIII and D-dimer were acutely elevated [2]. In this latter group, the frequency of PTS characterized by both physical and functional findings was 31%. The present finding of a cumulative incidence of physically and functionally significant PTS of only 13% at 1–2 years in a cohort of children sharing these adverse prognostic clinical and laboratory features indicates that PPMT deserves further evaluation as a potential means for PTS risk-reduction in this high-risk stratum of pediatric DVT.
The pathophysiology of PTS is thought to ultimately involve venous hypertension [20], which, in turn, is believed to derive from venous valvular reflux and/or persistent thrombotic veno-occlusion following DVT. The fact that PTS developed in several children in the present cohort in whom patency was both achieved acutely following PPMT and maintained long-term suggests that these patients may have irreversible valvular damage in the affected limb at the time of presentation or ongoing vascular inflammation following successful lysis. While plethysmography and other techniques of assessing venous valvular function were not performed in this cohort study, it should be recognized that PTS has been shown to correlate poorly with the presence of reflux by conventional methods [21]. As an alternative pathophysiological explanation to reflux secondary to mechanical valve damage, it is possible that valvular fibrosis and damage due to inflammation (whether systemic or localized to the vasculature) may be sufficient to cause insufficiency in these cases. Indeed, some evidence to date in adult DVT identifies elevated levels of interleukin-6 and D-dimer as prognostic of PTS [22,23]; the latter was a key inclusion criterion for cases in this cohort, based upon prior knowledge of prognostic factors for adverse outcomes of thrombosis in children [11].
Findings from a recent meta-analysis of randomized controlled trials (RCTs) of additional thrombolysis (systemic infusion or CDTI) versus standard anticoagulation alone revealed that, although mortality and the risk of recurrent VTE are not reduced by thrombolysis, the acute rate of complete patency is significantly increased (RR=4.14, 95% CI=1.22–14.01), and the risk of PTS is decreased (RR=0.66, 95% CI=0.47–0.94) [24]. However, a slight but statistically significant increase in acute major bleeding risk was ascribed to thrombolysis by systemic infusion/CDTI [RR=1.73, 95% CI=1.04–2.88]. Furthermore, the findings for PTS were based on only two RCTs in which this outcome was reported [25,26]. Recently (and not included in the aforementioned Cochrane review), early outcomes from the CaVenT trial indicate a significant risk reduction in chronic venous obstruction among adult patients with acute proximal DVT treated by additional CDTI when compared to those who received standard anticoagulation alone [27]. Evaluation for the primary endpoint of this trial, the rate of PTS at two years, is ongoing.
A potential advantage of the PMT/PPMT approach is the reduction in total dose of thrombolytic agent administered relative to thrombolysis by systemic infusion or CDTI, with a consequent theoretical benefit of decreased bleeding risk. Furthermore, PMT/PPMT offers the advantage of minimizing systemic lytic exposure in particular, thereby presumably decreasing systemic bleeding risks involving critical sites such as the central nervous system. No RCTs of PMT/PPMT have been published, and prospective data on this lytic intervention for DVT are limited to two adult studies focused upon CDTI, in which PMT/PPMT was reported in a few cases each [28,29]. By comparison, nine retrospective studies have been reported, totaling approximately 200 adult DVT patients treated by PMT/PPMT with/without adjunctive CDTI [30–37]. However, published experience with a regimen of PMT/PPMT followed by adjunctive CDTI is limited to three retrospective studies in adults, in which the number of patients receiving this regimen ranged between eight and 20 [30,33,34].
With regard to safety of PMT/PPMT with/without CDTI, only six cases of acute major bleeding and zero cases of acute symptomatic PE were reported among the nine retrospective studies, out of 172 and 107 patients, respectively, in whom this outcome was assessed. These low rates of peri-procedural hemorrhage and PE are consistent with our prospective findings in the present cohort. As for potential efficacy of PMT/PPMT, acute patency rates were high across the nine retrospective studies. However, long-term patency was only reported by Lin and colleagues [31], who determined a rate of 65%. No cases of acute recurrent DVT were reported among a cumulative total of 62 subjects in the retrospective studies wherein this outcome was assessed. Our findings of a higher rate of early local recurrent VTE in this prospective cohort could be explained by one or more of the following possibilities: (1) the risk of acute re-occlusion may be higher in children than adults; (2) observation biases may have led to under-reporting in the retrospective studies; (3) children in the present study were generally more prothrombotic than the adults reported in prior series. Given the latter possibility, and the low bleeding risks observed here, the present authors currently advocate for more aggressive peri-procedural anticoagulation than employed in these subjects. With regard to PTS, the findings reported here are the first among prospective or retrospective studies of PMT/PPMT in children or adults. Future analyses of PMT/PPMT must build upon this work, emphasizing PTS as a key outcome, and using validated measures.
Principal strengths of the present work include: the prospective inception cohort design; comprehensive clinical and laboratory evaluation for predisposing conditions (including venous stenoses and thrombophilia states); standardization of peri-procedural and extended anti-coagulant regimens; and PTS outcomes measurement, using a standardized instrument for which data on validity have been published in children. The principal limitations of the present observational study are its non-randomized design and its relatively small size. As in other non-randomized studies, selection biases may exist (e.g., clinical determination of PMT vs. PPMT and use/non-use of CDTI). Additionally, in spite of the fact that this is the largest pediatric series on PMT/PPMT, and similar in size to previous adult studies of PMT/PPMT in which adjunctive CDTI was frequently employed, it must be emphasized that our outcome estimates (e.g.: acute locally recurrent DVT, PE, and major hemorrhage; late recurrent VTE; PTS) may be imprecise given the size of our study population. In particular, PTS may develop over time, and may be underestimated here; yet, in prior work by the authors, for all children found to be affected at 2 years, PTS had been evident at 1 year post-event [2]. Given the study limitations of sample size, the tendencies observed for putative prognostic indicators of long-term patency and PTS must also be considered speculative. Therefore, larger prospective studies are required in order to confirm the safety of PMT/PPMT with adjunctive CDTI, evaluate its efficacy for PTS risk reduction, and define additional prognostic factors in this young patient population.
Acknowledgments
N.A.G. conceived and designed the research, collected data, analyzed data, interpreted findings, drafted the manuscript, and approved its final submission. B.B., M.W., and C.R. collected data, analyzed data, interpreted findings, critically revised the manuscript, and approved its final submission. J.D.D. and M.J.M.-J. conceived and designed research, analyzed data, interpreted findings, critically revised the manuscript, and approved its final submission.
The authors thank Timothy Nelson and Elizabeth Pounder for assistance in data collection. This work would not have been possible without the generous participation of the patients/families in this observational study.
Grant support: Dr. Goldenberg is funded in part by a Career Development Award from the National Institutes of Health, National Heart Lung and Blood Institute (1K23HL084055). Dr. Manco-Johnson is supported in part by a Cooperative Agreement for a Pilot Thrombosis/Thrombophilia Network from the Centers for Disease Control and Prevention (U01DD00016).
Footnotes
Conflicts of interest: None.
REFERENCES
- 1.Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P, Michelson AD. American College of Chest Physicians. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines(8th Edition) Chest. 2008;133 Suppl:887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg NA, Durham JD, Knapp-Clevenger R, Manco-Johnson MJ. A thrombolytic regimen for high-risk deep venous thrombosis may substantially reduce the risk of post-thrombotic syndrome in children. Blood. 2007;110:45–53. doi: 10.1182/blood-2006-12-061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg NA, Donadini MP, Kahn SR, Crowther M, Kenet G, Nowak-Göttl U, Manco-Johnson MJ. Post-thrombotic syndrome (PTS) in children: a systematic review of frequency of occurrence, validity of outcome measures, and prognostic factors. Haematologica. 2010 doi: 10.3324/haematol.2010.026989. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Chest. Vol. 133. 2008. American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines(8th Edition) pp. 454S–545S. Kearon et al., Chest 2008. [DOI] [PubMed] [Google Scholar]
- 5.Manco-Johnson MJ, Nuss R, Hays T, Krupski W, Drose J, Manco-Johnson ML. Combined thrombolytic and anticoagulant therapy for venous thrombosis in children. J Pediatr. 2000;136:446–453. doi: 10.1016/s0022-3476(00)90006-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Hays T, Balasa V, Bagatell R, Gruppo R, Grabowski EF, Valentino LA, Tsao-Wu G, Manco-Johnson MJ. Pediatric Coagulation Consortium. Low-dose tissue plasminogen activator thrombolysis in children. J Pediatr Hematol Oncol. 2003;25:379–386. doi: 10.1097/00043426-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Yee DL, Chan AK, Williams S, Goldenberg NA, Massicotte MP, Raffini LJ. Varied opinions on thrombolysis for venous thromboembolism in infants and children: findings from a survey of pediatric hematology-oncology specialists. Pediatr Blood Cancer. 2009;53:960–966. doi: 10.1002/pbc.22146. [DOI] [PubMed] [Google Scholar]
- 8.Kitchens CS. Thrombotic storm: when thrombosis begets thrombosis. Am J Med. 1998;104:381–385. doi: 10.1016/s0002-9343(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 9.Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, Janne d’Othée BM, Hofmann LV, Cardella JF, Kundu S, Lewis CA, Schwartzberg MS, Min RJ, Sacks S. Technology Assessment Committee of the Society of Interventional Radiology. J Vasc Interv Radiol. 2009;20:S391–S408. doi: 10.1016/j.jvir.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Manco-Johnson MJ, Grabowski EF, Hellgreen M, Kemahli AS, Massicotte MP, Muntean W, Peters M, Nowak-Göttl U. Laboratory testing for thrombophilia in pediatric patients. On behalf of the Subcommittee for Perinatal and Pediatric Thrombosis of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis (ISTH) Thromb Haemost. 2002;88:155–156. [PubMed] [Google Scholar]
- 11.Goldenberg NA, Knapp-Clevenger R, Manco-Johnson MJ. Elevated plasma factor VIII and D-dimer levels as predictors of poor outcome of thrombosis in children. N Engl J Med. 2004;351:1081–1088. doi: 10.1056/NEJMoa040161. [DOI] [PubMed] [Google Scholar]
- 12.Manco-Johnson MJ, Knapp-Clevenger R, Miller BI, Hays T. Post-thrombotic syndrome (PTS) in children: validation of a new pediatric outcome instrument and results in a comprehensive cohort of children with extremity deep vein thrombosis (DVT) Blood. 2003;102:553. Abstract. [Google Scholar]
- 13.Rutherford RB, Padberg FT, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity score: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–1312. doi: 10.1067/mva.2000.107094. [DOI] [PubMed] [Google Scholar]
- 14.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 15.Goldenberg NA, Pounder EP, Clevenger R, Manco-Johnson MJ. Post-thrombotic syndrome affecting the upper venous system in children: validation of outcome measurement and application in a single-institutional prospective inception cohort study. Blood. 2009;114:2979. Abstract. [Google Scholar]
- 16.Raffini L, Cahill AM, Hellinger J, Manno C. A prospective observational study of IVC filters in pediatric patients. Pediatr Blood Cancer. 2008;51:517–520. doi: 10.1002/pbc.21622. [DOI] [PubMed] [Google Scholar]
- 17.Murphy EH, Davis CM, Journeycake JM, DeMuth RP, Arko FR. Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May-Thurner Syndrome. J Vasc Surg. 2009;49 doi: 10.1016/j.jvs.2008.10.002. 697-73. [DOI] [PubMed] [Google Scholar]
- 18.Raffini L, Raybagkar D, Cahill AM, Kaye R, Blumenstein M, Manno C. May-Thurner syndrome (iliac vein compression) and thrombosis in adolescents. Pediatr Blood Cancer. 2006;47:834–838. doi: 10.1002/pbc.20728. [DOI] [PubMed] [Google Scholar]
- 19.Kuhle S, Koloshuk B, Marzinotto V, Zlotkin S, Burrows P, Ingram J, Adams M, Filler R. A cross-sectional study evaluating post-thrombotic syndrome in children. Thromb Res. 2003;111:227–233. doi: 10.1016/j.thromres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Kahn SR, Ginsberg J. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood Rev. 2002;16:155–165. doi: 10.1016/s0268-960x(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SR, Desmarais S, Ducruet T, Arsenault L, Ginsberg JS. Comparison of the Villalta and Ginsberg clinical scales to diagnose the post-thrombotic syndrome: correlation with patient-reported disease burden and venous valvular reflux. J Thromb Haemost. 2006;4:907–908. doi: 10.1111/j.1538-7836.2006.01824.x. [DOI] [PubMed] [Google Scholar]
- 22.Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post thrombotic syndrome. Thromb Haemost. 2009;101:505–512. [PubMed] [Google Scholar]
- 23.Latella J, Desmarais S, Kahn SR. Relationship between D-dimer level, venous valvular reflux, and development of the post-thrombotic syndrome after deep venous thrombosis. Blood. 2008;112:1821. doi: 10.1111/j.1538-7836.2010.04001.x. Abstract. [DOI] [PubMed] [Google Scholar]
- 24.Watson L, Armon M. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2004;3:CD002783. doi: 10.1002/14651858.CD002783.pub2. Updated 2007. [DOI] [PubMed] [Google Scholar]
- 25.Arnesen H, Heilo A, Jakobsen E, Ly B, Skaga E. A prospective study of streptokinase and heparin in the treatment of deep vein thrombosis. Acta Med Scand. 1978;203:457–463. doi: 10.1111/j.0954-6820.1978.tb14908.x. [DOI] [PubMed] [Google Scholar]
- 26.Schweizer J, Elix H, Altmann E, Hellner G, Forkmann L. Comparative results of thrombolysis treatment with rt-PA and urokinase: a pilot study. Vasa. 1998;27:167–171. [PubMed] [Google Scholar]
- 27.Enden T, Kløw NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbaek G, Sandset PM. CaVenT study group. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268–1275. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 28.Bjarnason H, Kruse JR, Asinger DA, Nazarian GK, Dietz CA, Jr, Caldwell MD, Key NS, Hirsch AT, Hunter DW. Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997;8:405–418. doi: 10.1016/s1051-0443(97)70581-5. [DOI] [PubMed] [Google Scholar]
- 29.Verhaeghe R, Stockx L, Lacroix H, Vermylen J, Baert AL. Catheter-directed lysis of iliofemoral vein thrombosis with use of rt-PA. Eur Radiol. 1997;7:996–1001. doi: 10.1007/s003300050239. [DOI] [PubMed] [Google Scholar]
- 30.Bush RL, Lin PH, Bates JT, Mureebe L, Zhou W, Lumsden AB. Pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis: safety and feasibility study. J Vasc Surg. 2004;40:965–970. doi: 10.1016/j.jvs.2004.08.025. Bush et al., J Vasc Surg 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lin PH, Zhou W, Dardik A, Mussa F, Kougias P, Hedayati N, Naoum JJ, El Sayed H, Peden EK, Huynh TT. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192:782–788. doi: 10.1016/j.amjsurg.2006.08.045. Lin et al., Am J Surg 2006. [DOI] [PubMed] [Google Scholar]
- 32.Gandini R, Maspes F, Sodani G, Masala S, Assegnati G, Simonetti G. Percutaneous ilio-caval thrombectomy with the Amplatz device: preliminary results. Eur Radiol. 1999;9:951–958. doi: 10.1007/s003300050775. [DOI] [PubMed] [Google Scholar]
- 33.Cynamon J, Stein EG, Dym RJ, Jagust MB, Binkert CA, Baum RA. A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol. 2006;17:1043–1049. doi: 10.1097/01.RVI.0000221085.25333.40. [DOI] [PubMed] [Google Scholar]
- 34.Jackson LSM, Xiu-Jie W, Dudrick SJ, Gersten GD. Catheter-directed thrombolysis and/or thrombectomy with selective endovascular stenting as alternatives to systemic anticoagulation for treatment of acute deep vein thrombosis. Am J Surg. 2005;190:864–868. doi: 10.1016/j.amjsurg.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Shi H-J, Huang Y-H, Shen T, Qiang X. Percutaneous mechanical thrombectomy combined with catheter-directed thrombolysis in the treatment of symptomatic lower exremity deep venous thrombosis. Eur J Radiol. 2009;71:350–355. doi: 10.1016/j.ejrad.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Protack CD, Bakken AM, Patel N, Saad WE, Waldman DL, Davies MG, et al. Long-term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement. J Vasc Surg. 2007;45:992–997. doi: 10.1016/j.jvs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Parikh S, Motarjeme A, McNamara T, Raabe R, Hagspiel K, Benenati JF, Sterling K, Comerota A. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008;19:521–528. doi: 10.1016/j.jvir.2007.11.023. [DOI] [PubMed] [Google Scholar]