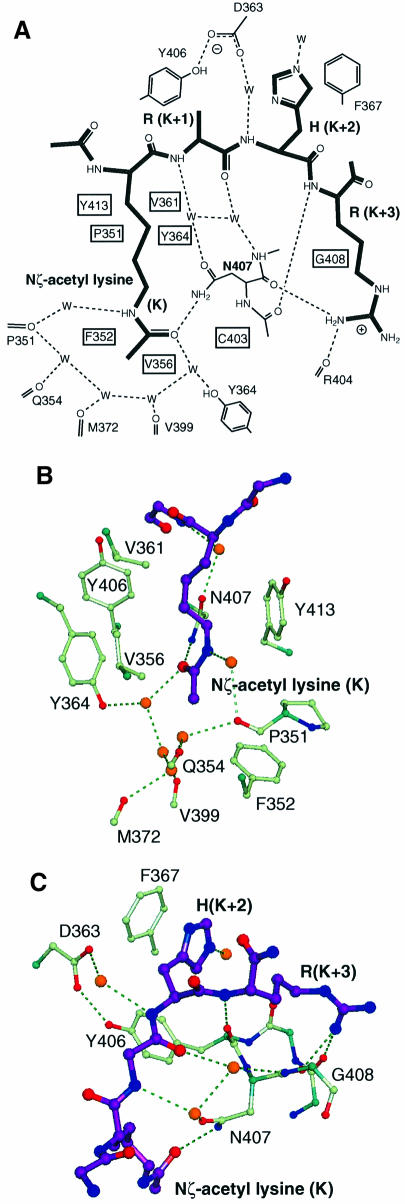
Fig. 5. Details of the peptide binding site. (A) Schematic view of interactions, with water molecules represented as W. (B) The N-acetyl lysine slot showing the ring of water molecules around the acetyl group at the base of the slot, and the hydrophobic walls left and right. (C) The binding groove for the (K + 2) and (K + 3) peptide residues lies across the 405–408 loop between αB and αC. His(K + 2) packs against Phe367, and Arg(K + 3) forms hydrogen bonds back to the protein backbone. The peptide backbone forms four hydrogen bonds to the protein, three of them via water molecules.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.