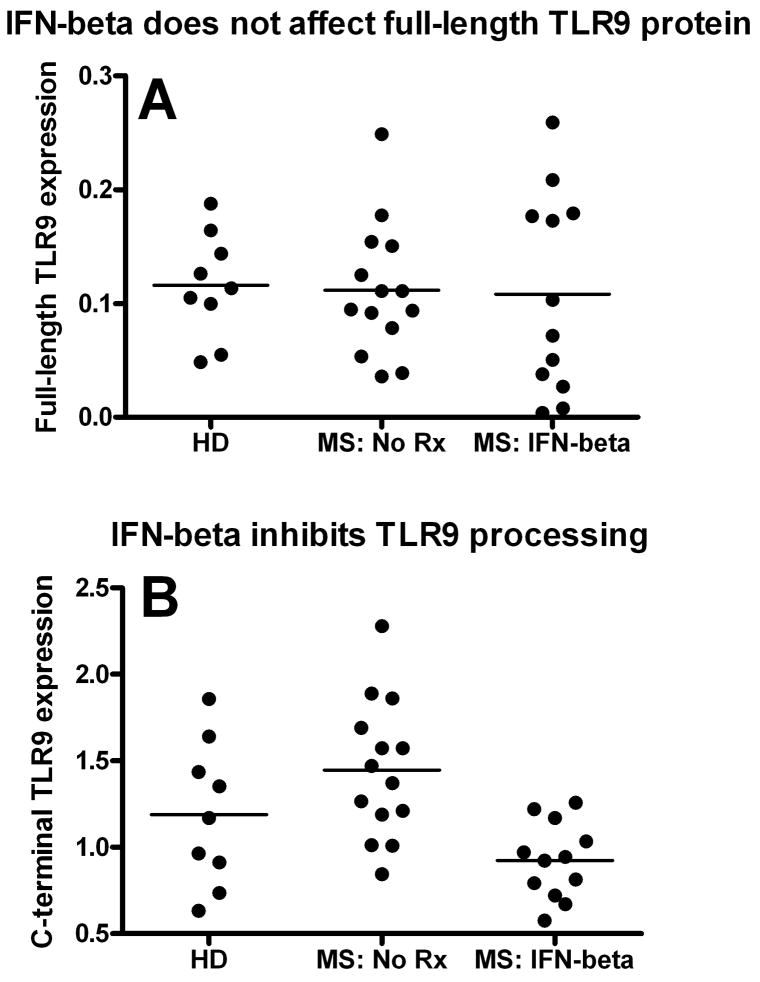
Figure 4.
IFN-beta treatment inhibits TLR9 processing in MS. Plasmacytoid dendritic cells (pDCs) were separated from healthy donors (HD), nontreated MS/CIS patients (MS: No Rx), and MS/CIS patients treated with IFN-beta–based medications (MS: IFN-beta) for at least 3 months. The patient’s characteristics are presented in Table 2. The level of the (A) full-length and (B) processed TLR9 protein expression was determined in the same patients by western blot, as described in Patients and Methods. IFN-beta-treated CIS/MS patients had a similar level of the full-length TLR9 (mean ± SEM = 0.108 ± 0.025 relative units, n = 12) compared to untreated patients (0.112 ± 0.015 relative units, n = 14). However, IFN beta-treated patients had a significantly decreased level of the processed TLR9 C-terminal when compared to untreated patients (mean ± SEM = 0.924 ± 0.063 vs 1.445 ± 0.106 relative units, respectively; p = 0.0005). The level of the TLR9 C-terminal in healthy subjects was 1.189 ± 0.1385 relative units (n = 9), which was less than, but not statistically different from, untreated patients. SEM = standard error of the mean.