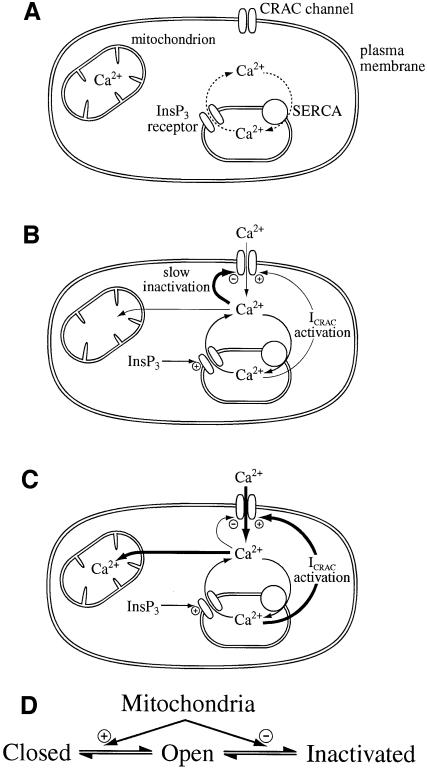
Fig. 5. Cartoon summary of mitochondrial role in ICRAC in weak (physiological) intracellular Ca2+ buffer. (A) Shows the resting state, where ICRAC is not functioning. Stores are full and any Ca2+ that leaks from the stores is re-sequestrated by the SERCA pumps. (B) Following an increase in the levels of the second messenger InsP3 in the absence of active mitochondrial Ca2+ uptake, Ca2+ is released from the stores. However, the SERCA pumps are able to re-sequestrate sufficient Ca2+ to prevent the threshold for macroscopic activation of ICRAC from being reached. Only a very small fraction of CRAC channels are activated (undetectable in whole-cell mode). Furthermore, the rise in cytosolic Ca2+ results in strong inactivation of ICRAC through Ca2+-dependent slow inactivation. (C) In the presence of respiring mitochondria, InsP3 activates macroscopic ICRAC. Ca2+ released from the stores by InsP3 is taken up by mitochondria through a ruthenium red-sensitive uniporter. This reduces the amount of Ca2+ available to the SERCA pumps and in the vicinity of open InsP3 receptors, such that the stores are depleted sufficiently for macroscopic ICRAC to activate (less refilling by SERCA pumps and less inactivation of InsP3 receptors). ICRAC now activates. Some refilling does occur because inclusion of thapsigargin enhances the size of the current. Furthermore, mitochondrial Ca2+ buffering reduces the rate and extent of Ca2+-dependent slow inactivation, thereby increasing the size and duration of the current. (D) A simplified gating scheme for CRAC channels summarizing the role of mitochondrial Ca2+ buffering. Mitochondria facilitate opening (Closed to Open transition) whilst simultaneously reducing inactivation (Open to Inactivated transition). In this way, mitochondria have a much larger impact on ICRAC than through either transition alone.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.