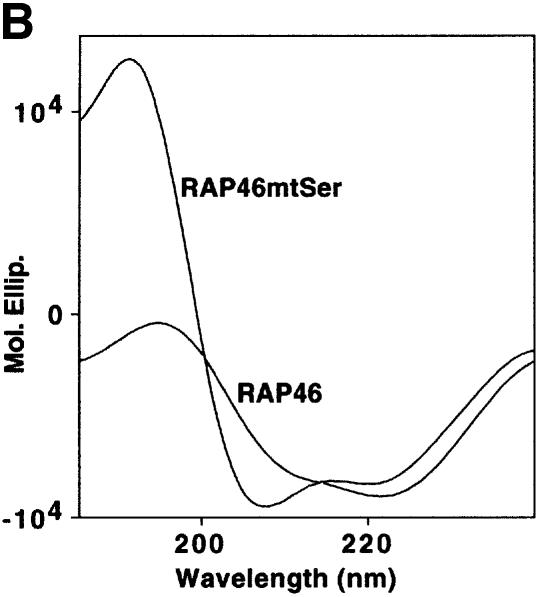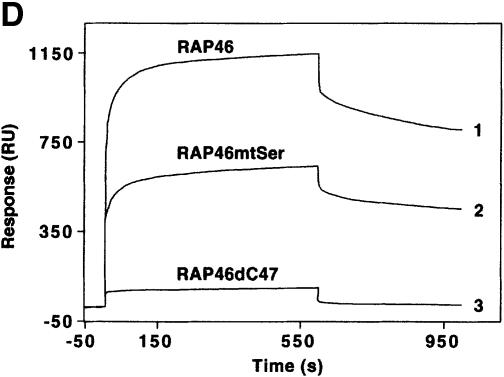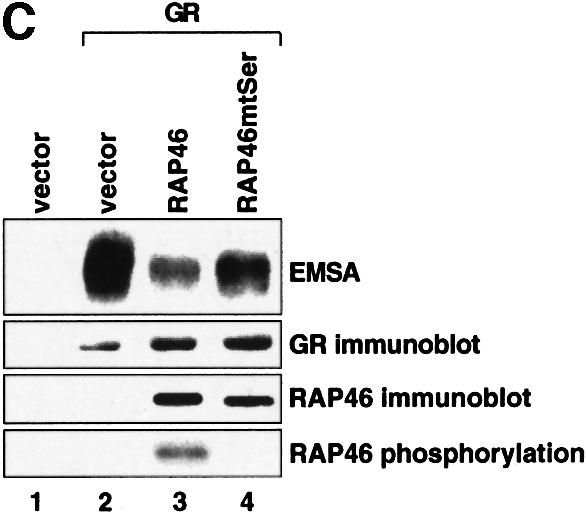
Fig. 1. Mutation of the serine residues at the N-terminus of RAP46 does not abolish the inhibitory effect of RAP46 on DNA binding by the GR. (A) The sequences of the first 70 amino acids of the wild-type and the N-terminal serine mutant of RAP46 are illustrated. The amino acids exchanged are indicated by boxes. (B) Mutation of the serine residues at the N-terminus of RAP46 alters the secondary structure of the protein. Purified, bacterially produced fusion proteins GST–RAP46 and GST–RAP46mtSer were subjected to circular dichroism measurements. (C) Mutation of the N-terminal serine residues does not alter the negative effect of RAP46 on DNA binding by the GR. A hundred thousand COS-7 cells were transiently transfected with 0.9 µg of either an empty expression vector (vector) or an expression vector encoding glucocorticoid receptor (GR) and 0.9 µg of expression vectors encoding HA-tagged RAP46 or the mutant RAP46mtSer. One half of the transfected cells was used for EMSA and the other half was metabolically labelled with [32P]orthophosphate for 4 h and immunoprecipitated with an anti-HA antibody. The immunoprecipitated proteins were resolved by SDS–PAGE and exposed to autoradiography (RAP46 phosphorylation) or immunoblotted using antibodies recognizing either the GR (GR immunoblot) or RAP46 as well as RAP46mtSer (RAP46 immunoblot). (D) Surfaces with an average of 2000 resonance units (RU) immobilized GST–RAP46 fusion proteins were obtained by covalent immobilization of an anti-GST antibody with the help of a standard amine coupling protocol, followed by capture of the different fusion proteins through antibody–antigen interaction. Injection of Hsp70 over the surface resulted in a strong interaction signal by 100 ng GST–RAP46 (1), whereas a decrease in the interaction signal was obtained when 100 ng GST–RAP46mtSer (2) were used for capture. No interaction was detected with 100 ng GST–RAP46dC47 (3).