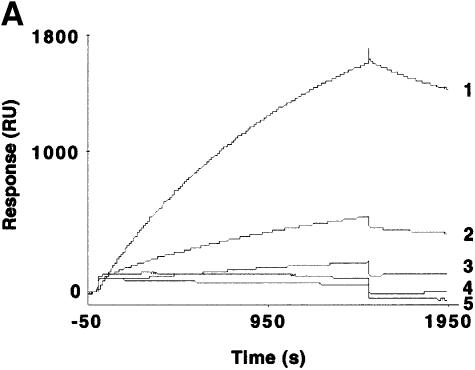
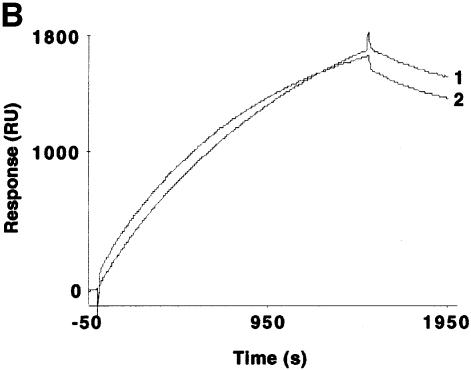
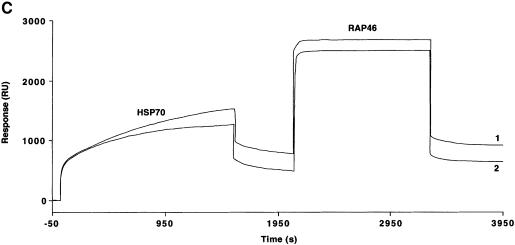
Fig. 3. Hsp70 binds to the GR in the absence or presence of hormone and its binding is required for the interaction of RAP46 with GR in SPR. (A) Sensograms showing injections of Hsp70 at 100 (1), 50 (2), 25 (3), 12.5 (4) and 5 ng/µl (5) with 1500 RU His-tagged GR produced in baculovirus coupled to the surface of a NTA chip. A dose-dependent increase in resonance units (Response, RU) can be seen. (B) SPR signals obtained when 100 ng of Hsp70 were injected in the absence (1) or presence (2) of 10–7 M dexamethasone and 1500 RU His-tagged GR coupled to the NTA chip. A minor decrease in the interaction is observed in the presence of dexamethasone. (C) The consecutive addition of 100 ng RAP46 to a preformed GR–Hsp70 complex in the absence (1) or presence (2) of 10–7 M dexamethasone resulted in an additional increase in RU, indicative of RAP46 binding. The values of the apparent rate constants for the Hsp70–GR interaction deduced from the experiments described in (A) were Ka = 5.4 × 102 M/s and Kd = 8.23 × 10–4/s. From these data the apparent dissociation equilibrium constant was calculated to be Kd = 1.5 × 10–6 M. All calculations were based on the assumption of monomolecular 1:1 binding characteristics, where A + B = AB. However, the apparent rate and dissociation equilibrium constants have to be seen as first approximations since a steady drift in the baseline (i.e. a time-dependent decline in GR binding; not shown) was observed. This is a well known phenomenon when working with non-covalent capturing of His-tagged proteins to the surface of a NTA sensor chip.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.