Figure 2.
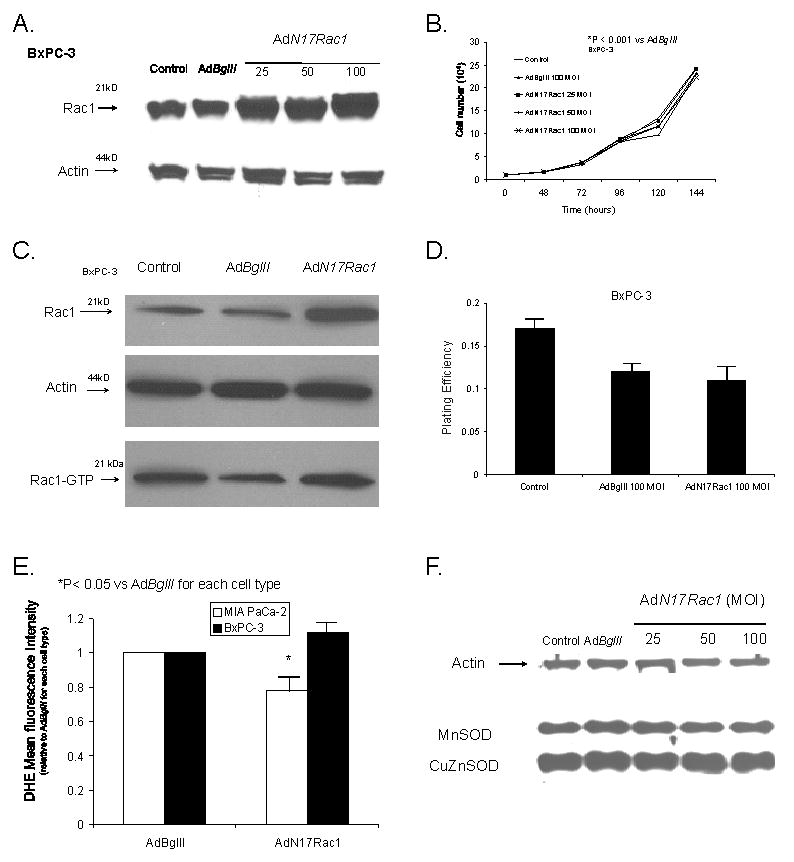
AdRac1N17 infection increased Rac1 protein levels but had no effect on growth in BxPC-3 cells. A. Cells transduced with 0–100 MOI AdRac1N17 demonstrated increases in Rac1 immunoreactivity with increasing viral titer. No difference was seen with AdBglII transfer compared with parental cells.
B. Overexpression of AdRac1N17 had no effect on BxPC-3 cell growth. BxPC-3 cells transduced with 0 100MOI AdRac1N17 or 100 MOI AdBglII demonstrated no changes in cell growth with AdRac1N17100 MOI. No significant changes were seen with AdBglII transduction compared with parental cells. Mean in vitro cell growth of control, AdRac1N17-or AdBglII-transduced BxPC-3 cells are shown. Each point represents the mean values, n= 3. *P < 0.05 vs. 100 MOI AdBglII.
C. Infection with AdRac1N1 increased Rac1 immunoreactivity but did not decrease Rac1 activity. Representative western blot showing increases in Rac1 protein but no change in Rac1 activation in AdRac1N17-transduced BxPC-3 cells.
D. Plating efficiency was unchanged with AdRac1N17 infection in BxPC-3 cells. BxPC-3 cells were infected with AdRac1N17 or AdBglII and then plated for clonogenic survival. AdRac1N17 infected cells did not change plating efficiency at 100 MOI when compared to AdBglII infected cells. Each point represents the mean values, n = 3.
E. Superoxide levels decreased in MIA PaCa-2 cells after infection with the AdN17Rac1 construct without changes in antioxidant proteins that scavenge superoxide. Intracellular hydroethidine fluorescence decreased in MIA PaCa-2 cells but not BxPC-3 cells infected with AdN17Rac1. Intracellular superoxide levels as measured by DHE decreasedsignificantly 48 hours after infection with AdN17Rac1 100 MOI compared to MIA PaCa-2 pancreatic carcinoma cells infected with the AdBglII vector. *p < 0.05 vs AdBglII, means ± SEM, n=3.
F. Overexpression of AdN17Rac1 did not change immunoreactive protein for MnSOD or CuZnSOD in MIA PaCa-2 cells. MIA PaCa-2 cells were transfected with 100 multiplicity of infection (MOI) of AdBglII as a negative control or 0 to 100 MOI of AdN17Rac1. After 48 hr of transfection, total cell lysate was prepared and immunoblotted for the proteins with cellular actin as a loading control. Total protein was electrophoresed in a 12.5% SDS-polyacrylamide running gel and a 5% stacking gel. After blocking in 20% fetal bovine serum for 1 hr, the sheets were washed and then treated with antibodies to MnSOD and CuZnSOD.
