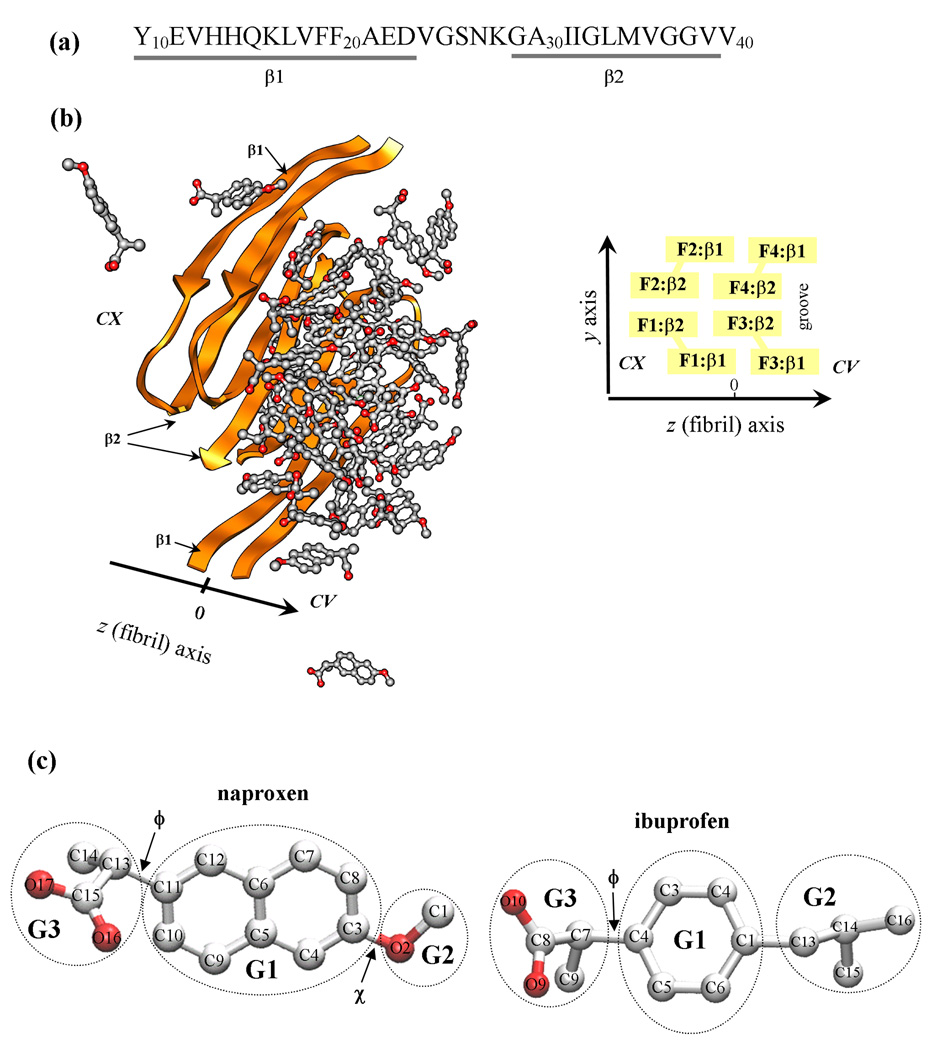
Fig. 1.
(a) The sequence of Aβ10–40 peptide and the allocation of the β1 and β2 β-strands formed in the fibril structure. (b) Aβ10–40 fibril with bound naproxen molecules. Fibril protofilament is built of four stacked β-sheets formed by the β1 and β2 strands (panel (a)). A groove formed by indented β2 sheets results in the appearance of two distinct fibril edges - concave (CV) and convex (CX). The CX and CV edges are formed by the peptides F1,F2 and F3,F4, respectively. The primary binding site for naproxen and ibuprofen is the groove on the CV edge. (c) Naproxen molecule has a central hydrophobic naphthalene ring (group G1) and two polar moieties, methoxy and carboxylate groups (G2 and G3). The dihedral angles ϕ and χ are used to test parameterization of naproxen. Ibuprofen molecule has three structural moieties - hydrophobic phenyl G1 and isobutyl G2 and hydrophilic carboxylate G3 groups. The dihedral angle ϕ in ibuprofen is analogous to that in naproxen. Carbon and oxygen atoms are shown in grey and red.