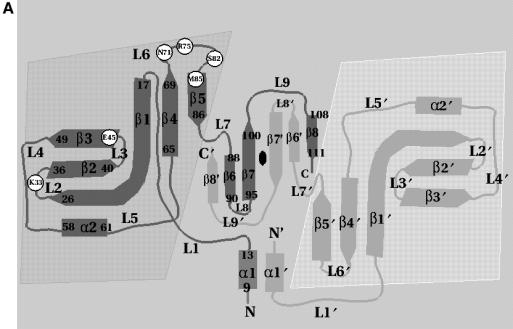
Fig. 2. (A) Schematic representation of the topology of Trbp111. For clarity, starting and ending residues of secondary structure elements are shown on one monomer only. Shaded areas denote the OB-fold domains and the central region is the dimerization domain. Circles are positions of alanine substitutions that diminish tRNA binding. Secondary structure nomenclature corresponds to the A.aeolicus sequence. (B) Sequence alignment of A.aeolicus Trbp111 and its E.coli homolog showing identities (vertical lines) and similarities (dots). Sites of alanine substitutions that diminish or do not affect tRNA binding are highlighted in dark and light gray, respectively.

