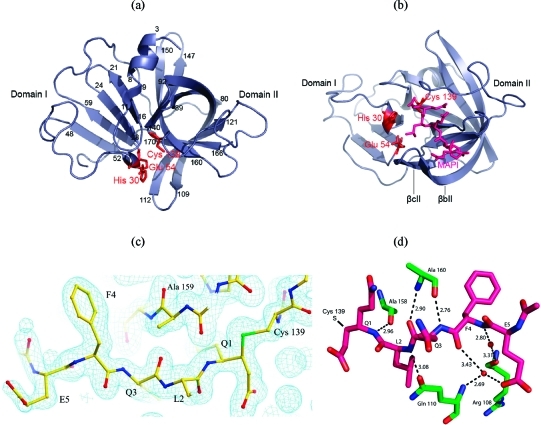
Figure 4.
(a) SV3CP viewed through the β-barrel of domain II. The putative active site catalytic triad of residues Cys 139, His 30, and Glu 54 is indicated in red. Residues delineating the secondary structure elements are indicated numerically. The conformation of the inhibitor MAPI bound to Cys 139 is shown in violet in (b). The β-strands βbII and βcII create an arch that binds with the peptidyl portion of MAPI (corresponding to the natural substrate P residues). (c) Electron density for the inhibitor MAPI. The residues of the inhibitor are shown along with the 2Fo − Fc electron density at 1.7 Å resolution contoured at 1 rms. The contiguous electron density between Q1 and Cys 139 (shown on the far right) is evident; the side-chain sulfur atom is colored green. (d) The β-sheet-like hydrogen-bonding network between the active site cleft residues of SV3CP and the inhibitor MAPI. The SV3CP residues are shown in green and those of MAPI in pink. A number of water-mediated hydrogen bonds are also shown, and donor−acceptor atom distances are given in Å.