Table 2.
Antibacterial and antifungal activities of pyridinium oximes
| IC50 (MIC) µg/mL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | 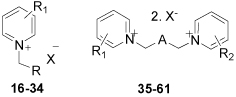 |
Antibacterial |
Antifungal |
||||||
| S. aureus | MRS |
M. intracellulare |
C. neoformans |
C. glabrata |
A. fumigatus |
||||
| 21 | R = 5-bromothiophen-2-yl, R1 = 2-CHNOH, X = Cl |
15.27 | 14.19 | NA | na | >20 | na | ||
| 24 | R = benzothiophen-3-yl, R1 = 2-CHNOH, X = Cl |
12.23 (20) |
17.72 (20) | >20 | na | na | na | ||
| 25 | R = benzothiophen-3-yl, R1 = 3-CHNOH, X = Cl |
na | na | na | na | na | >20 | ||
| 33 | R = 3-methyl-isoxazol-5-yl, R1 = 3-CHNOH, X = Br |
na | na | na | >20 | na | na | ||
| 45 | R1 = 4-CONH2, R2 = 2- CHNOH, A = CH2CH2, X = Br |
na | na | na | >20 | na | na | ||
| 46 | R1 = 4-CONH2, R2 = 3- CHNOH, A = CH2, X = Br |
na | na | na | >20 | na | na | ||
| 50 | R1 = R2 = 2-CHNOH, A = thiophen-2,5-yl, X = Cl |
na | na | na | >20 | na | na | ||
| 51 | R1 = R2 = 3-CHNOH, A = thiophen-2,5-yl, X = Cl |
na | na | na | >20 | na | na | ||
| 52 | R1 = R2 = 4-CHNOH, A = thiophen-2,5-yl, X = Cl |
na | na | na | >20 | na | na | ||
| 53 | R1 = 4-CONH2, R2 = 4- CHNOH, A = thiophen-2,5- yl, X = Cl |
na | na | na | >20 | na | na | ||
| 58 | R1 = R2 = 4-CONH2, A = furan-2,5-yl, X = Br |
na | na | na | >20 | na | na | ||
| 60 | R1 = R2 = 3-CHNOH, A = isoxazol,3,5-yl, X = Br |
na | na | na | >20 | na | na | ||
| 61 | R1 = R2 = 4-CHNOH, A = isoxazol,3,5-yl, X = Br |
na | na | na | >20 | na | na | ||
| Ciprofloxacin | 0.1 (0.5) | 0.09 (0.25) |
0.39 (0.5) | nt | nt | nt | |||
| Amphotericin B | nt | nt | nt | 1.28 (2.5) | 0.7 (1.25) |
0.98 (2.5) | |||
IC50, the concentration that affords 50% inhibition of bacterial/ fungal growth; na, not active; nt, not tested; MIC (Minimum inhibitory concentration) is the lowest concentration (µg/ml) that allows no detectable growth; *None of the compound was active against C. albicans, C. krusei, E. coli and P. aeruginosa (data not shown).
