Figure 3. Systemic administration of recombinant group V sPLA2 ameliorates K/BxN serum-transfer arthritis.
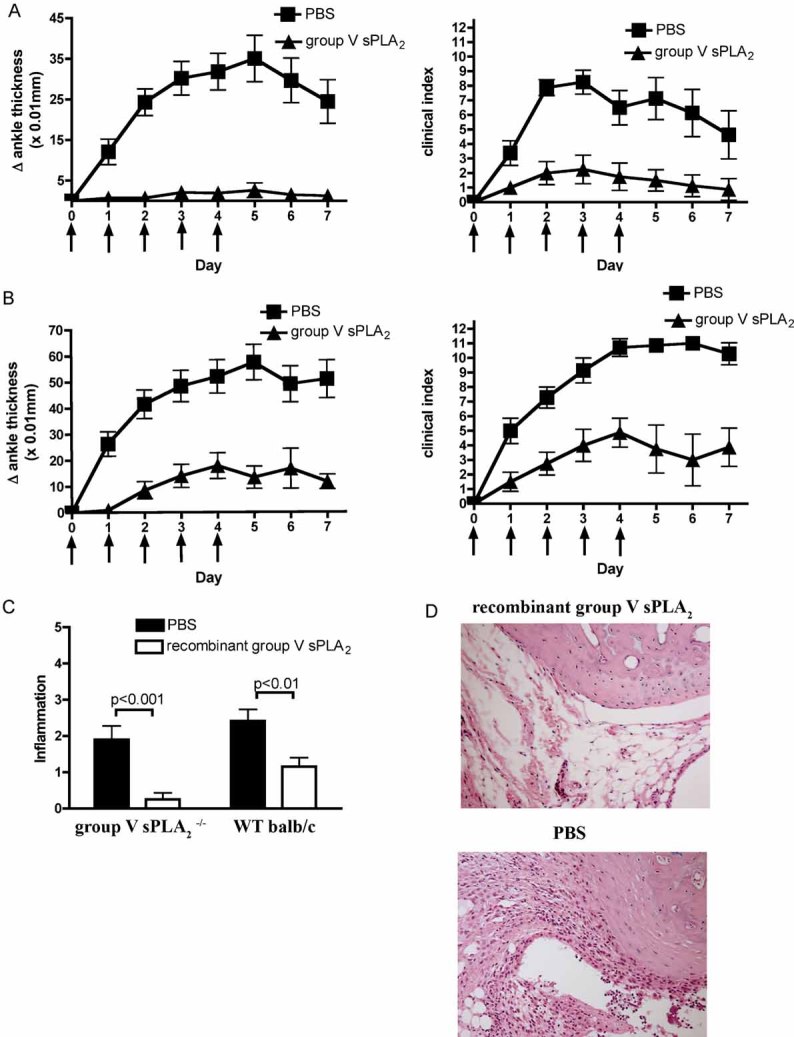
- Recombinant mouse group V sPLA2 (50 µg in PBS) or control PBS were injected intravenously into group V sPLA2-null mice in a BALB/c group IIA null background 2 hours prior to administration of K/BxN serum on experimental Day 0 and daily thereafter for 5 days. Arthritis was induced by injection of 35 µl and 20 µl of K/BxN serum on day 0 and 2 respectively; the development of arthritis was monitored for 7 days. Arrows indicate sPLA2-V intravenous injections.
- Experiment as detailed in (A) using WT BALB/c mice
- Histomorphometric quantification of inflammation in ankle sections from mice injected with recombinant group V sPLA2 or PBS control at experimental day 7. Note that the BALB/c mice express both group V and group IIA sPLA2 and therefore are not the congenic controls to group V sPLA2−/−. N = 12 mice/group. Data are mean ± SEM pooled from three independent experiments. P < 0.001 for (A and B).
- Representative mid-saggital ankle sections from group V sPLA2 null mice treated with recombinant group V sPLA2 or its diluent (PBS). Note the decreased leukocytic infiltration, synovial lining hyperplasia and pannus formation in recombinant group V sPLA2 treated mice. Magnification = 200×. Figures are representative of findings in 12 mice/group.
