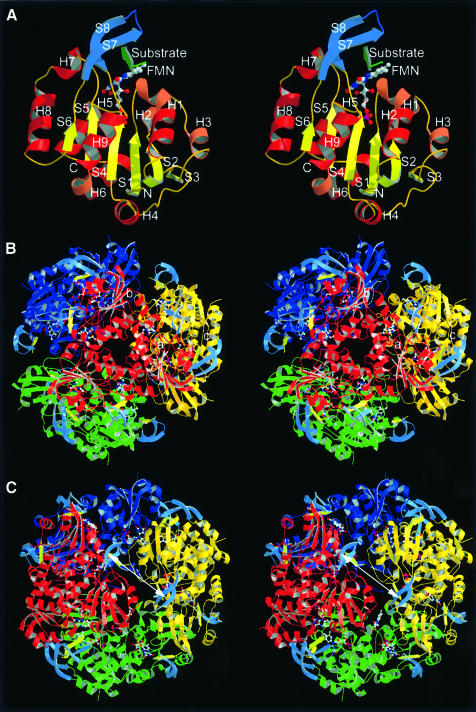
Fig. 2. Monomer, trimer and dodecamer structure of EpiD. (A) Monomer structure. β-S1 to S6 in yellow, α-helices in dark red and 310-helices in light red. The substrate peptide (in green) is embraced by the substrate binding clamp (S7 and S8 in blue). (B) View along the 3-fold axis. The FMN cofactor is buried in the centre of the trimer interface. The top rim near the trimer axis is formed by α-helix H8. (C) View along the 2-fold axis. The substrates are shown in light yellow. The distance between the two active sites is ∼31 Å (Sγ to Sγ) indicated by a white arrow.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.