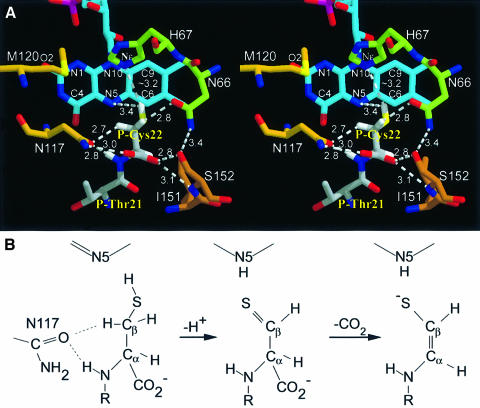
Fig. 7. Active site architecture and proposed mechanism. (A) The active site base His67 was modelled into the substrate complex after superposition of FMN and hydrogen atoms were generated in the most favoured conformation. Ile68 packing between His67 and P-Cys22 was omitted for clarity (see Figure 6B). Cβ hydrogen atoms are in hydrophilic environments, the Cα hydrogen atom is shielded by a hydrophobic interaction with Ile151. The cis geometry of the enethiol product suggests a motion of Sγ towards N5. The carboxylate group is fixed by H-bonds between Asn117 and Ser152 of the peptide clamp, the resulting carbon dioxide can exit freely. (B) Proposed mechanism. The starting geometry suggests oxidation of Sγ yielding a thioaldehyde intermediate and its spontaneous decarboxylation forming a double bond between Cα and Cβ reconstituting the thiol group.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.