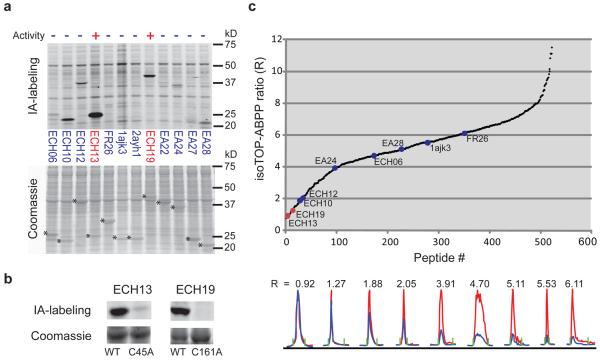
Figure 5. Quantitative reactivity profiling predicts functional cysteines in designed proteins.
a, In-gel fluorescence demonstrates robust IA labeling of two active cysteine hydrolases, ECH13 and ECH19 , relative to inactive designs (top panel). Hydrolysis activities of ECH13 and ECH19 measured as the ratio of velocities in the presence versus absence of purified enzymes were 71.64+/−6.94 and 104.15+/−10.78, respectively (see Supplementary Fig. 15a for substrate hydrolysis assay). Other designs showed no measurable hydrolysis activity over background (0.76 +/− 0.058 nmol/s). Asterisks designate Coomassie blue signals for protein designs (lower panel). b, IA-labeling is observed for ECH13 and ECH19, but not their active-site cysteine mutants C45A and C161A, respectively. c, Catalytic cysteines in ECH13 and ECH19 demonstrate low isoTOP-ABPP ratios (red) compared with other designs (blue). Chromatographs are shown for peptides from the nine designs identified in this experiment (bottom panel).