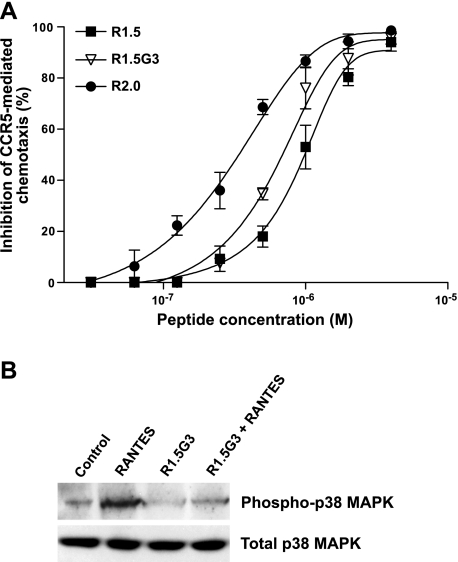
Figure 6.
CCR5-antagonistic activity of RANTES peptide mimetics. A) Dose-response antagonistic effects of peptides R1.0, R1.5G3, and R2.0 on T-cell chemotaxis. Chemotaxis was assessed on the human CD4+CD195+ T-cell clone PM1 (16) stimulated with RANTES (25 nM). As a proof of specificity of the assay, we demonstrated that the CCR5-specific inhibitor Maraviroc (100 nM) completely abrogated the RANTES-elicited chemotactic migration of PM1 cells (not shown). Chemotaxis was measured as previously described (9) using Transwell plates with a membrane pore size of 5.0 μm. The number of cells migrated into the lower chamber in replicate wells was measured by timed flow cytometry (60 s) at a flow rate of 120 μl/min (high) using a FACSCanto A cytometer (Becton Dickinson). Specific cell migration was calculated by subtracting the number of cells migrated in the absence of the chemotactic factor from the number of cells migrated in the presence of the chemotactic factor; chemotaxis inhibition by peptides was calculated as the percentage of cells migrated toward RANTES in the absence of the peptide. Data represent averages ± sd from ≥3 independent experiments; absolute values of cell migration ranged from 1815 to 2780 cells/well in controls (in the absence of RANTES) and from 5800 to 10,430 cells/well in the presence of RANTES (without inhibitors). B) Agonistic and antagonistic effects of peptide R1.5G3 on p38 MAPK phosphorylation. Semiconfluent PM1 cultures, pretreated or not with peptide R1.5G3 at 5 μM, were stimulated for 20 min with RANTES (25 nM) or left unstimulated. Levels of total and phosphorylated p38 MAP kinase were evaluated by Western blot on cell lysates.