Abstract
Objectives
To determine the electrophysiologic effects of simvastatin in canine pulmonary vein (PV) sleeve preparations.
Background
Ectopic activity arising from the PV plays a prominent role in the development of atrial fibrillation (AF).
Methods
Transmembrane action potentials were recorded from canine superfused left superior or inferior PV sleeves using standard microelectrode techniques. Acetylcholine (ACh, 1 μM), isoproterenol (1 μM), high calcium ([Ca2+]o=5.4mM) or a combination was used to induce early or delayed afterdepolarizations (EADs or DADs) and triggered activity. Voltage clamp experiments were performed in the left atrium measuring fast and late sodium currents.
Results
Under steady-state conditions, simvastatin (10 nM, n=9) induced a small increase in action potential duration measured at 85% repolarization (APD85) and a significant decrease in action potential amplitude, take-off potential, and maximum rate of rise of action potential upstroke at the fastest rates. Vmax decreased from 175.1 ± 34 to 151.7 ± 28 V/s and from 142 ± 47 to 97.4 ± 39 V/s at basic cycle lengths of 300 and 200 ms, respectively. Simvastatin (10-20 nM) eliminated DADs and DAD-induced triggered activity in 7 of 7 PV sleeve preparations and eliminated or reduced late-phase 3 EADs in 6 of 6 PV sleeve preparations. Simvastatin (20 nM) did not affect late or fast sodium currents.
Conclusions
Our data suggest that in addition to its upstream actions to reduce atrial structural remodeling, simvastatin exerts a direct antiarrhythmic effect by suppressing triggers responsible for the genesis of AF.
Keywords: Atrial fibrillation, Antiarrhythmic drugs, Sodium channel blocker, Electrophysiology, Pharmacology, Statins
Introduction
Since their discovery in Japan in the mid 1970s, 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) have been widely used to reduce low density lipoprotein (LDL) and total cholesterol. Other beneficial cardiovascular effects, in addition to those involving the lipid profile, have been attributed to statins. These so-called pleiotropic effects are mainly related to the anti-inflammatory and potential antiarrhythmic effects of statins, particularly in the setting of atrial fibrillation (AF).1 Experimental studies have shown that statins attenuate atrial electrical remodeling induced by atrial tachypacing and can thus prevent the creation of the substrate for development of AF.2 Simvastatin was shown to greatly attenuate the effect of tachypacing to downregulate the L-type Ca2+ channel α-subunit (Cav1.2) expression. A number of randomized and large observational clinical studies have reported beneficial effects of statins in AF, particularly in the setting of heart failure. Treatment with statins was linked to a decreased incidence or recurrence of AF in patients with a history of AF, those undergoing cardiac surgery, post-ablation, those implanted with a pacemaker or ICD, or in patients with acute coronary syndrome.3-7 To the best of our knowledge no controlled clinical studies have been performed evaluating the acute effect of simvastatin on atrial fibrillation
Ectopic activity arising from the pulmonary veins (PV) has been shown to play an important role in the development of AF.8 Late-phase 3 early afterdepolarizations (EADs) as well as delayed afterdepolarizations (DADs)-induced triggered activity originating from PV sleeves have been proposed as potential triggers in the initiation of AF.9-15
The present study was designed to test the hypothesis that simvastatin's ameliorative effects on AF are in part mediated by its actions to suppress triggered activity arising from PV sleeves.
Methods
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No 85-23, Revised 1996) and was approved by the ACUC of the Masonic Medical Research Laboratory.
Adult mongrel dogs, weighing 20-35 kg, were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, IV). The chest was opened via a left-thoracotomy and the heart excised and placed in a cold cardioplegic solution ([K+]0 = 8 mmol/L, 4°C).
Superfused pulmonary vein sleeve preparation
PV sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from left canine atria. The canine pulmonary vein sleeve model consists of a small rectangular section of cardiac muscle, approximately 2 cm by 1.5 cm and 1-2 mm thick. The preparation is isolated from the left atrium adjacent to the pulmonary vein. The vein is cut open so that muscle and vein lay flat, thus exposing the surface area from which to record action potentials under control conditions and following exposure to the different solutions used in the study. The tissue is superfused with normal Tyrode solution or Tyrode solution containing simvastatin, isoproterenol, high calcium, or their combinations. Therefore the different drugs diluted in the Tyrode solution are not directly perfused through the pulmonary vein and should not experience mechanical interference through competitive flow.
Similarly to the findings in the human heart,16 histological studies performed in the canine heart have shown that sleeves of endocardium penetrate the proximal section of the pulmonary vein.17, 18 In this study, action potentials were recorded from the endocardial extension of atrial muscle covering the pulmonary veins. Left superior pulmonary veins were used preferentially in most experiments. The preparations were placed in a small tissue bath and superfused with Tyrode's solution of the following composition (mM): 129 NaCl, 4 KCl, 0.9 NaH2PO4, 20 NaHCO3, 1.8 CaCl2, 0.5 MgSO4, 5.5 glucose, buffered with 95% O2/5% CO2 (35 ± 0.5°C). PV preparations were stimulated at a basic cycle length (BCL) of 1000 ms during the equilibration period (1h) using electrical stimulation (1-3 ms duration, 2.5 times diastolic threshold intensity) delivered through silver bipolar electrodes insulated except at the tips. Transmembrane potentials were recorded using glass microelectrodes filled with 3 M KCl (10-20 MΩ DC resistance) connected to a high input-impedance amplification system (World Precision Instruments, model KS-700, New Haven, CT). The following parameters were measured: take-off potential (TOP), action potential amplitude (APA), action potential duration at 85% repolarization (APD85), and maximal rate of rise of action potential upstroke (Vmax). Transmembrane action potentials were recorded at a sampling rate of 41 kHz. Measurement of TOP was used instead of resting membrane potential (RMP) because the slow phase 3 of the action potential of the PV sleeve preparation fails to return to the resting potential at the fastest rates.
Isolated myocyte preparation
Atrial myocytes were prepared from canine hearts using techniques described previously.19 Briefly, male and female adult mongrel dogs were anesthetized with sodium pentobarbital (35mg/kg i.v.) their hearts were rapidly removed and placed in nominally Ca2+-free Tyrode's solution. The left atria was excised, cannulated via the ostium and perfused with nominally Ca2+-free Tyrode's solution containing 0.1% BSA for a period of about 5 minutes. The atria was then subjected to enzyme digestion with the nominally Ca2+-free solution supplemented with 0.5 mg/ml collagenase (Type II, Worthington) and 1 mg/ml BSA for 30 minutes. After perfusion, tissue from the appendage and the pectinate muscle were isolated, placed in separate beakers, minced and incubated in fresh buffer containing 0.5 mg/ml collagenase, 1 mg/ml BSA and agitated. The supernatant was filtered, centrifuged and the pellet containing the myocytes was stored at room temperature.
Voltage Clamp Recordings of Peak INa
Early sodium current, INa, was measured as previously described19 with minor modifications. Experiments were performed using a MultiClamp 700A (Axon Instruments, Foster City, CA). Command voltages were delivered and data acquired via a DigiData 1322 computer interface using Axon Instruments' pClamp 9 program suite with data stored on computer hard disk. Patch pipettes were pulled from borosilicate glass (1.5 mm o.d. and 1.1 mm i.d.) on a Model PP-830 vertical puller (Narashige Instruments, Japan). The electrode resistance was 1-2.5 MΩ when filled with the internal solution (see below). The membrane was ruptured by applying negative pressure and series resistance compensated by 75 to 80%. Whole cell current data was acquired at 20-50 kHz and filtered at 5 kHz. Currents were normalized to cell capacitance. All peak INa experiments were performed at 15° C.
External solution contained (in mM): NMDG 105, NaCl 40, CaCl2 2.0, MgCl2 1, glucose 10, HEPES 10, pH adjusted to 7.4 with HCl. The pipette solution contained (mM): NaCl 5, aspartate 120, MgCl2 1, CsCl 5, HEPES 10, MgATP 5, EGTA 10, pH adjusted to 7.2 with CsOH. Peak sodium current was dramatically reduced in the low extracellular sodium to ensure adequate voltage control. Cells were held at -90 mV or -120 mV before evoking a train of 15 100-ms pulses to -30 mV with a diastolic interval of 100-ms.
Voltage Clamp Recordings of Late INa
Late INa density was a measured in full external Na+ at 37° C as previously described.20 External solution contained (in mM): NaCl 140, CaCl2 2.0, MgCl2 1, glucose 10, HEPES 10, pH adjusted to 7.4 with NaOH. The pipette solution contained (mM): NaCl 10, aspartate 130, MgCl2 1, CsCl 10, HEPES 10, MgATP 5, EGTA 10, pH adjusted to 7.2 with CsOH.
Late INa density was recorded in cells that were held at -90 mV. Currents were recorded during a train of 15 pulses. To remove steady-state inactivation, a 160-ms or 200-ms pulse to -120 mV was taken before a 200-ms pulse to -30 mV. Simvastatin at concentrations of 20 nM or 5 uM was applied and the train repeated, followed by 10 uM TTX. Late INa, characterized as the TTX-sensitive difference current, was measured as the total charge moved beginning 15 ms after the start of the step to -30.
Whole cell currents were analyzed using the Clampfit analysis program from pClamp 9 suite (Axon Instruments).
Drugs
Simvastatin was dissolved in dimethyl sulfoxide to form a stock solution of 100 uM and used at a final concentration of 10 to 20 nM. Isoproterenol (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in distilled water to form a stock solution of 1 mM and used at final concentrations of 1 uM and 0.5 uM. It should be noted that a previous study performed in rabbit myocytes 21 used “activated” simvastatin 22 to measure calcium as well as transient outward currents. We used “activated” simvastatin to measure sodium currents and repeated experiments evaluating the effects of “activated” simvastatin in PV sleeve preparations. Similar results were observed with “activated” and “non-activated” forms of simvastatin, therefore the data were pulled together in the results section. Acetylcholine (ACh) was dissolved in distilled water to form a stock solution of 10 mM and used at a concentration of 1 uM.
Statistics
Electrophysiological data are presented as mean ± SD. Statistical analysis of action potential data obtained in the presence and absence of drug was performed using paired t-test and statistical comparisons of voltage-clamp data were made using ANOVA followed by a Student-Newman Keuls test. Mean values were considered to be statistically different at p < 0.05.
Results
Effects of simvastatin on pulmonary vein (PV) sleeve preparations
Simvastatin (10 nM) accentuated the depolarization and reduction of take-off potential normally observed in PV sleeve preparations with rapid rates of activation (Figure 1). The more positive take-off potential at the faster rates is due to a small simvastatin-induced increase in the duration of action potential caused by a slowing of the final part of phase 3. Composite data show a small increase in action potential duration measured at 85% repolarization (APD85) and a significant decrease in action potential amplitude (APA), take-off potential (TOP), and maximum rate of rise of action potential upstroke (Vmax) at the fastest rates (Table 1, Figure 2). Our findings indicate that simvastatin induces a marked use-dependent depression of Vmax in PV sleeve preparations.
Figure 1.
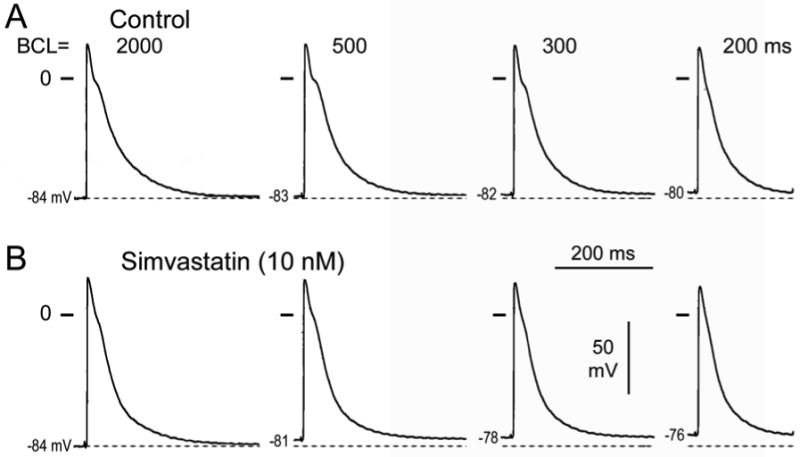
Effect of simvastatin in a pulmonary vein (PV) sleeve preparation. A. Control action potentials recorded at basic cycle lengths (BCLs) of 2000, 500, and 300 ms. B. Effect of simvastatin (10 nM). Simvastatin accentuated the depolarization and reduction of take-off potential observed in the PV sleeve at fast rates.
Table 1.
Rate-dependent effects of simvastatin (Sim, 10 nM) on electrophysiological parameters. APD85 : action potential duration at 85 % repolarization; APA: action potential amplitude; TOP : take-off potential. Vmax: maximum rate of rise of action potential upstroke. BCL: basic cycle length.
| BCL (ms) | 2000 | 1000 | 500 | 300 | 200 | |
|---|---|---|---|---|---|---|
| APD85 (ms) | C Sim |
160.1 ± 20 | 150.5 ± 14 | 135.8 ± 17 | 119.1 ± 23 | 104.3 ± 23 |
| 162.8 ± 24 | 153.7 ± 19 | 141.1 ± 17 | 126.2 ± 13 | 112.1 ± 15 | ||
| APA(mV) | C Sim |
111.2 ± 7 | 110.8 ± 6 | 109.2 ± 6 | 106.0 ± 6 | 99.8 ± 5 |
| 109.4 ± 8 | 108.4 ± 7 | 107.2 ± 7 | 102.9 ± 8 | 91.0 ± 9* | ||
| TOP(mV) | C Sim |
-81.8 ± 4 | -81.2 ± 4 | -80.5 ± 3 | -78.6 ± 4 | -76.8 ± 3 |
| -81.5 ± 5 | -80.2 ± 5 | -78.1 ± 4* | -75.5 ± 4* | -71.6 ± 6* | ||
| Vmax (V/s) | C Sim |
211.1 ± 35 | 209.4 ± 36 | 197.4 ± 35 | 175.1 ± 34 | 142 ± 47 |
| 211.0 ± 36 | 204.6 ± 36 | 183.6 ± 30* | 151.7 ± 28* | 97.4 ± 39* | ||
p<0.05 simvastatin vs. control (C). N=9.
Figure 2.
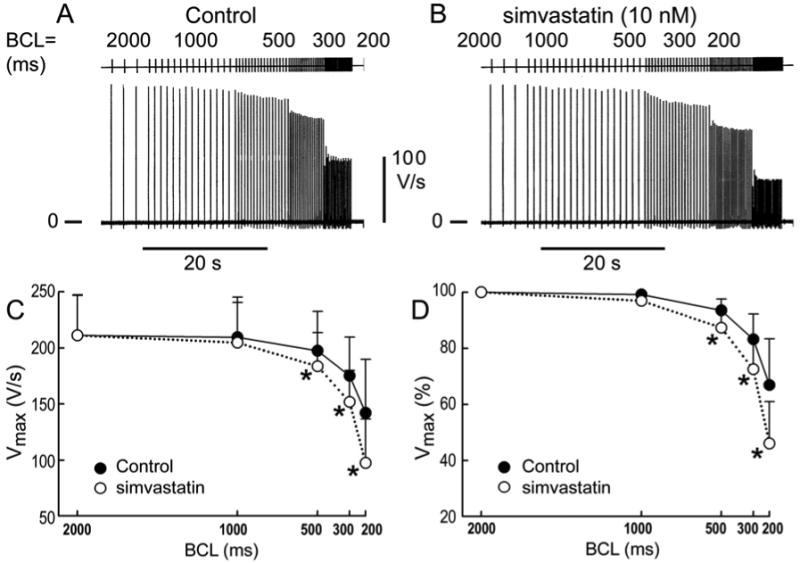
Rate-dependent effects of simvastatin on Vmax in PV sleeve preparations. A. Control recording of Vmax recorded at basic cycle lengths (BCLs) of 2000, 1000, 500, 300, and 200 ms in a PV sleeve preparation. B. Effect of simvastatin (10 nM). C. Composite data (n=9) of effects on Vmax expressed in absolute values. D. Effects on Vmax expressed as values normalized to a BCL of 2000 ms. Acceleration from a BCL of 2000 to 300 ms led to a 16.8% decrease of Vmax under control conditions and a 27.5% decrease following addition of simvastatin (p<0.05). A change in BCL from 2000 to 200 ms produced a 33.1% decrease under control conditions and a 54 % decrease upon addition of simvastatin (p<0.05).
Effects of simvastatin on delayed and early afterdepolarizations (DADS and EADs)-induced triggered activity
In another series of experiments, we determined the effects of simvastatin on DADs, late-phase 3 EADs and triggered activity elicited by exposure of the PV sleeve preparations to acetylcholine (ACh), isoproterenol, high [Ca2+], or their combination and rapid pacing. Simvastatin (10-20 nM) eliminated DADs and DAD-induced triggered activity in 7 of 7 PV sleeve preparations (Figure 3). Simvastatin (10 to 20 nM) eliminated late phase 3 EADs and triggered activity in 6 of 6 PV sleeve preparations (Figure 4). Simvastatin also suppressed ACh (1 uM) and high calcium (5.4 mM) induced late phase 3 EADs observed in alternate beats at rapid pacing rates (Figure 5).
Figure 3.
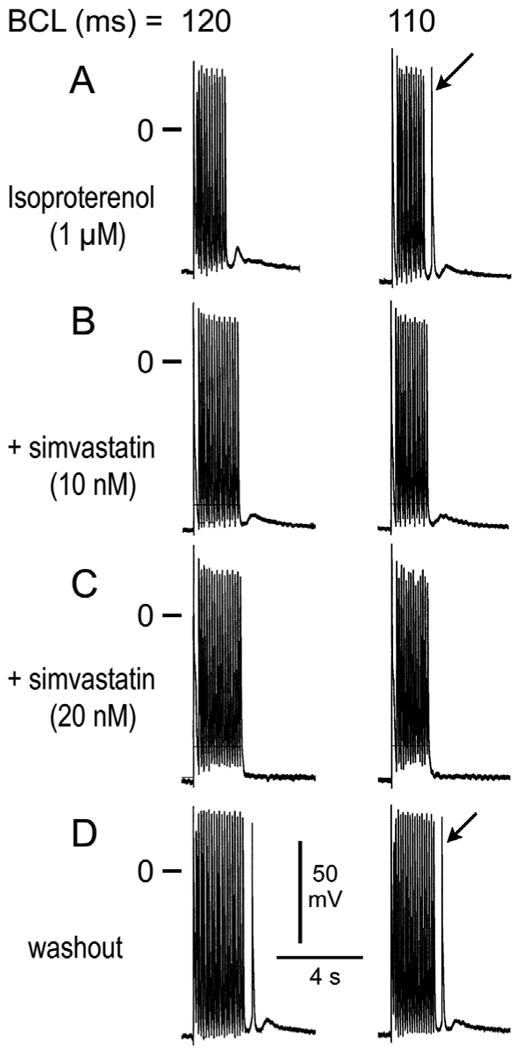
Effect of simvastatin to suppress isoproterenol-induced delayed afterdepolarizations (DADs) and triggered activity. A. Induction of DAD and triggered response following a train of 20 beats at basic cycle lengths (BCLs) of 120 and 110 ms, respectively, in the presence of isoproterenol. B. Simvastatin (10 nM) eliminates the triggered beat and reduces DAD amplitude. C. Simvastatin (20 nM) eliminates the triggered beat as well as DAD activity. D. Washout of simvastatin restores triggered activity.
Figure 4.
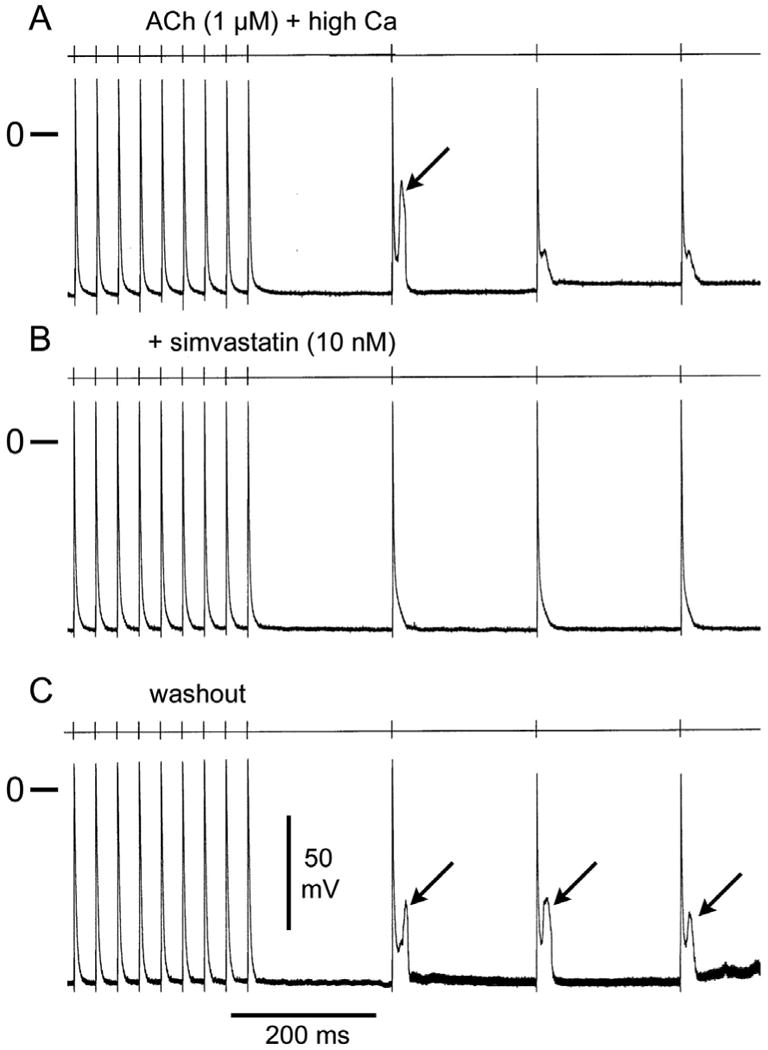
Simvastatin eliminates late-phase 3 early afterdepolarizations (EADs) and triggered activity. A. Effect of acetylcholine (ACh, 1 uM) and high calcium (5.4 mM) to induce late-phase 3 EADs and triggered activity following a train of 20 beats at a basic cycle length (BCL) of 200ms. B. Simvastatin (10 nM) suppresses EADs and triggered activity. C. Washout of simvastatin restores triggered activity.
Figure 5.
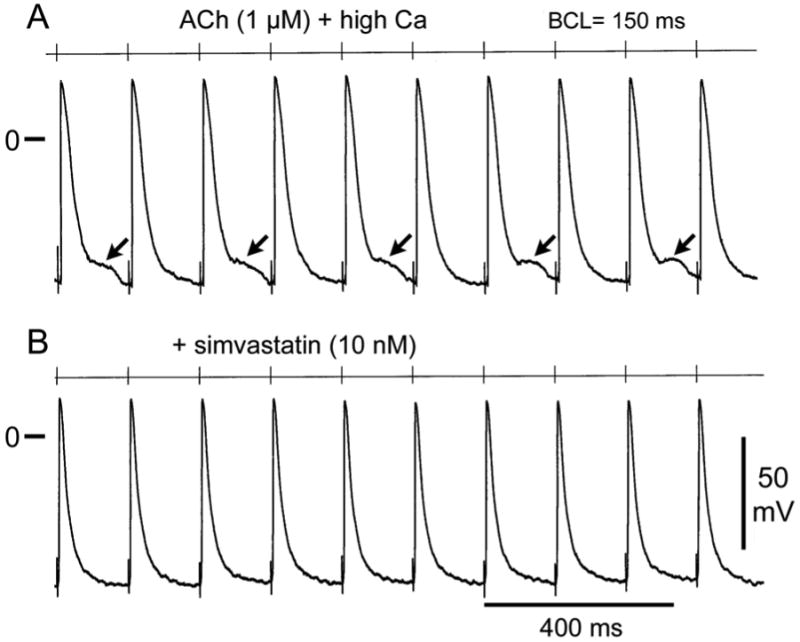
Effect of simvastatin to suppress late phase 3 early afterdepolarizations (EADs) elicited at rapid stimulation rates (BCL= 150 ms). A. Acetylcholine (ACh, 1 uM) and high calcium (5.4 mM) induce late phase 3 EADs in alternate beats. B. Simvastatin (10 nM) eliminates late phase 3 EADs.
Effects of simvastatin on late and fast sodium currents
Figure 6 shows that INa, late was unaffected by 5 uM simvastatin. Panel A shows INa, late in control solutions and 4 minutes after applying the drug. While exposed to the drug, the diastolic interval was abbreviated from 200 ms to 160 ms, and the currents in panel B were recorded. In the presence of 5 uM simvastatin, INa, late was unaffected by shortening the diastolic interval. Figure 7 summarizes the experiments showing that early and late INa were unaffected by 20 nM simvastatin.
Figure 6.
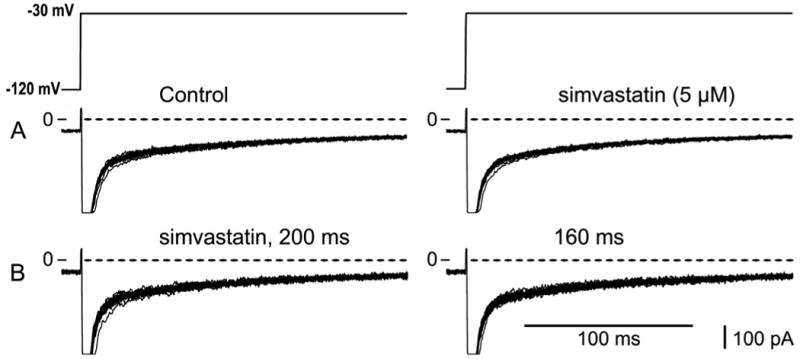
Late INa unaffected by simvastatin. A. Currents recorded during a train of 15 pulses in control solution (left) and in 5 uM simvastatin (right) at a diastolic interval of 200 ms. B. In the presence of 5 uM simvastatin, rate dependence of drug was investigated by shortening the diastolic interval to 160 ms.
Figure 7.
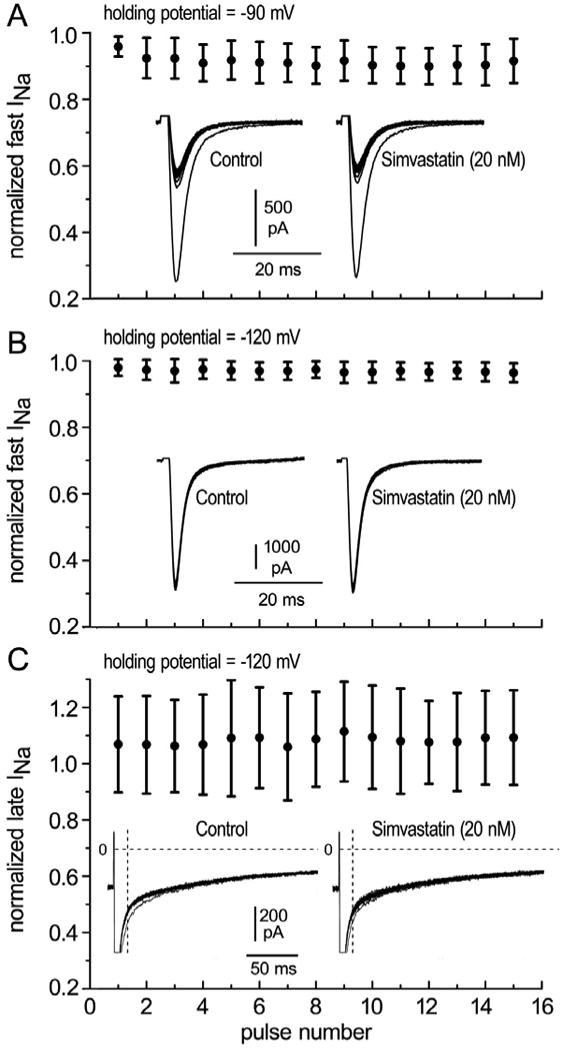
Early and late INa in the presence of 20 nM simvastatin. Currents were normalized to the corresponding current recorded in control solution. A. Normalized fast INa as a function of pulse number recorded from a holding potential of -90 mV (panel A, n=11 atrial cells, p>0.05) or -120 mV (panel B, n=11 atrial cells, p>0.05). C. Late INa in the presence of 20 nM simvastatin as a function of pulse number (n=11 atrial cells, p>0.05). Late INa was measured as the area delimited by the dotted lines.
Discussion
AF is the most common arrhythmia requiring medical attention. Ectopic activity arising from the PV sleeves is thought to be an important source of triggers and in some cases substrate for the development of AF.23-25 The results of the present study indicate that concentrations of simvastatin within the therapeutic range (8-20 nM)17, 26 induce rate-dependent depression of Vmax in canine PV sleeve preparations. Our results also indicate that simvastatin suppresses DADs, late-phase 3 EADs, and triggered activity induced in PV sleeves by the addition of isoproterenol, acetylcholine, high calcium, or their combination. These data suggest a direct antiarrhythmic effect of simvastatin in addition to the previously described upstream therapeutic effect.
Simvastatin eliminates EAD and DAD-induced triggered activity in PV sleeves
The present study shows that late-phase 3 EADs and DADs can be easily induced in PV sleeve preparations following the addition of isoproterenol, ACh, and high calcium, alone or in combination. Similar findings were previously reported by Chen and co-workers in canine and rabbit isolated single PV myocytes,17, 27 by Patterson and co-workers in isolated canine pulmonary vein sleeve preparations,12, 13 and by Burashnikov and co-workers in coronary-perfused right atrial preparations.11, 28 In isolated myocytes, isoproterenol was shown to increase automaticity and induce spontaneous as well as EAD or DAD-induced triggered activity.17, 27 Late-phase 3 EADs and late-phase 3 EAD-induced triggered activity11, 28 is a relatively new concept of arrhythmogenesis. Abbreviated repolarization permits normal sarcoplasmic reticulum calcium release and associated sodium-calcium exchange inward current to induce a late-phase 3 EAD, resulting in closely coupled triggered responses when it reaches threshold. Conditions permitting intracellular calcium loading facilitate the development of late-phase 3 EADs. Patterson and co-workers showed that autonomic stimulation could give rise to this phenomenon in canine PV sleeves and in some cases result in a run of triggered responses.12, 13
Our data illustrate that simvastatin in concentrations of 10 and 20 nM (within its therapeutic range) is able to eliminate late-phase 3 EADs and DAD-induced triggered activity arising from these two mechanisms. Similar findings were reported for ranolazine, an anti-anginal agent with a potent effect to cause use-dependent depression of sodium channel activity at rapid rates, similar to that of simvastatin.9
Ionic mechanisms
In isolated mouse ventricular myocytes, simvastatin has been shown to inhibit the late ICa, although at much higher concentrations (IC50 = 50 μmol/L).21 The data point to a lack of an effect of simvastatin on either early (peak) and late sodium currents in canine atrial cells despite a significant effect of the drug to reduce Vmax in PV sleeves at fast rates (BCL of 500, 300 and 200 ms). The decrease in Vmax may be explained by a simvastatin-induced depolarization and a more positive take-off potential at the faster rates due to a small simvastatin-induced increase in the duration of action potential caused by a slowing of the final part of phase 3. Additional studies will be necessary to establish the ionic basis for this change.
Suppression of DAD and late-phase 3 EAD activities by simvastatin can be explained by its actions to reduce intracellular sodium loading, which occurs at rapid activation rates. By doing so, simvastatin reduces calcium loading which normally occurs under these conditions as a result of sodium-calcium exchange.29-31 The effects may also been explained by an effect of simvastatin to block calcium current.21
Comparison of simvastatin with traditional antiarrhythmic drugs
The antifibrillatory action of most antiarrhythmic agents used to treat AF has been ascribed chiefly to their ability to induce prolongation of the effective refractory period (ERP), by prolonging APD (i.e., dl- sotalol and dofetilide), reducing excitability (i.e., flecainide and propafenone), or by a combined mechanism (i.e., amiodarone and ranolazine). In experimental studies, multiple ion-channel blockers, like amiodarone and ranolazine, act by prolonging ERP both by increasing APD as well as by producing a marked depression of excitability, thus inducing post-repolarization refractoriness especially at rapid activation rates (use-dependent effect). 32, 33
Compared to these traditional antiarrhythmic agents, simvastatin produces little change in APD, but does induce a depression of Vmax and excitability at rapid rates, although not as accentuated as in the case of chronic amiodarone34, 35. This action of simvastatin is most similar to that of Class IB antiarrhythmic agents such as lidocaine. The effects of simvastatin on EADs and DAD-induced triggered activity are similar to those of ranolazine.9 Chronic amiodarone has also been shown to prevent the development of EADs, DADs, and triggered activity induced by exposure to isoproterenol, ACh, high calcium or their combination.10, 35 Thus, the actions of simvastatin on the triggers of AF (DAD or EAD-induced triggered activity) are similar to those of traditional antiarrhythmic drugs, whereas its effects on the substrate are less pronounced. These findings suggest that further studies examining the efficacy of a combination of statins with antiarrhythmic agents in the management of AF, may be warranted.
Clinical implications: antiarrhythmic effects of statins
Statins are the most common drugs used in the clinical treatment of hypercholesterolemia. Patients administered statins for their lipid-lowering actions may benefit from their upstream effects,36 which are related to anti-inflammatory actions as well as their direct effects to minimize electrical remodeling.37
Clinical38 and experimental studies point to a prominent role of pulmonary veins in the genesis of atrial arrhythmias, including AF.8, 27 Our data suggest that in addition to its upstream effects and direct actions to reduce electrical remodeling during rapid activation of the atria, simvastatin can suppress late-phase 3 EADs, DADs and triggered activity induced in PV sleeves. Interestingly, other agents with upstream actions like the antihypertensive agents losartan and enalapril have also been shown in preliminary reports to eliminate DADs and late phase 3 EAD-induced triggered activity in PV sleeves.39 Our findings suggest that simvastatin, and perhaps other statins, may exert an important antiarrhythmic effect to suppress the triggers that contribute to the development of atrial fibrillation. Further clinical studies are warranted to evaluate the role of satins in the prevention and treatment of atrial arrhythmias, including atrial fibrillation.
Conclusion
Our data suggest that in addition to its upstream actions to reduce atrial structural remodeling, simvastatin exerts a direct antiarrhythmic effect by suppressing triggers responsible for the genesis of AF.
Acknowledgments
Supported by grant HL47678 from NHLBI (CA) and NYS and Florida Grand Lodges F. & A.M..
Abbreviations / Acronyms
- ACh
Acetylcholine
- AF
atrial fibrillation
- APD85
action potential duration measured at 85 % repolarization
- BCL
basic cycle length
- DADs
delayed afterdepolarizations
- EADs
early afterdepolarizations
- ICa
calcium current
- INa
sodium current
- late INa
late sodium current
- PV
pulmonary vein
- Vmax
maximal rate of rise of action potential upstroke
Footnotes
There is no conflict of interest.
References
- 1.Hadi HA, Mahmeed WA, Suwaidi JA, Ellahham S. Pleiotropic effects of statins in atrial fibrillation patients: the evidence. Vasc Health Risk Manag. 2009;5:533–551. doi: 10.2147/vhrm.s4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–2319. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- 3.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini CN, Vittinghoff E, Lin F, Hulley SB, Marcus GM. Statin use is associated with lower risk of atrial fibrillation in women with coronary disease: the HERS trial. Heart. 2009;95:704–708. doi: 10.1136/hrt.2008.154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphries KH, Lee M, Sheldon R, Ramanathan K, Dorian P, Green M, Kerr CR. Statin use and recurrence of atrial fibrillation after successful cardioversion. Am Heart J. 2007;154:908–913. doi: 10.1016/j.ahj.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Liakopoulos OJ, Choi YH, Kuhn EW, Wittwer T, Borys M, Madershahian N, Wassmer G, Wahlers T. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J Thorac Cardiovasc Surg. 2009;138:678–686. doi: 10.1016/j.jtcvs.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Li L, Korantzopoulos P, Liu E, Li G. Statin use and development of atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. 2008;126:160–170. doi: 10.1016/j.ijcard.2007.07.137. [DOI] [PubMed] [Google Scholar]
- 8.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 9.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2009;20:803–810. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Wongcharoen W, Chen YC, Chen YJ, Chen SY, Yeh HI, Lin CI, Chen SA. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm. 2007;4:1338–1349. doi: 10.1016/j.hrthm.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Lo LW, Chen YC, Chen YJ, Wongcharoen W, Lin CI, Chen SA. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharmacol. 2007;571:197–208. doi: 10.1016/j.ejphar.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Hocini M, Ho SY, Kawara T, Linnenbank AC, Potse M, Shah D, Jais P, Janse MJ, Haissaguerre M, de Bakker JM. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation. 2002;105:2442–2448. doi: 10.1161/01.cir.0000016062.80020.11. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 18.Fynn SP, Kalman JM. Pulmonary veins: anatomy, electrophysiology, tachycardia, and fibrillation. Pacing Clin Electrophysiol. 2004;27:1547–1559. doi: 10.1111/j.1540-8159.2004.00675.x. [DOI] [PubMed] [Google Scholar]
- 19.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol. 2001;281:H689–H697. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- 21.Vaquero M, Caballero R, Gomez R, Nunez L, Tamargo J, Delpon E. Effects of atorvastatin and simvastatin on atrial plateau currents. J Mol Cell Cardiol. 2007;42:931–945. doi: 10.1016/j.yjmcc.2007.03.807. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghi MM, Collinge M, Pardi R, Bender JR. Simvastatin modulates cytokine-mediated endothelial cell adhesion molecule induction: involvement of an inhibitory G protein. J Immunol. 2000;165:2712–2718. doi: 10.4049/jimmunol.165.5.2712. [DOI] [PubMed] [Google Scholar]
- 23.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 24.Nattel S, Allessie MA, Haissaguerre M. Spotlight on atrial fibrillation-the ‘complete arrhythmia’. Cardiovasc Res. 2002;54:197–203. doi: 10.1016/s0008-6363(02)00324-3. [DOI] [PubMed] [Google Scholar]
- 25.Nattel S. Combined parasympathetic-sympathetic nerve discharge and pulmonary vein afterdepolarizations: A new unifying concept with basic and clinical relevance. Heart Rhythm. 2005;2:632–633. doi: 10.1016/j.hrthm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 27.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 28.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 29.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Goodrow RJ, Jr, Scornik F, Perez G. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Therapeut. 2004;9 1:S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 30.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92 4:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Shryock J, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 32.Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. Ann N Y Acad Sci. 2010;1188:78–86. doi: 10.1111/j.1749-6632.2009.05086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burashnikov A, Antzelevitch C. New development in atrial antiarrhythmic drug therapy. Nat Rev Cardiol. 2010;7:139–148. doi: 10.1038/nrcardio.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sicouri S, Moro S, Litovsky SH, Elizari MV, Antzelevitch C. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol. 1997;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 35.Sicouri S, Burashnikov A, Belardinelli L, Antzelevitch C. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol. 2010;3:88–95. doi: 10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smit MD, Van G I. Upstream therapy of atrial fibrillation. Expert Rev Cardiovasc Ther. 2009;7:763–778. doi: 10.1586/erc.09.59. [DOI] [PubMed] [Google Scholar]
- 37.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 38.Nattel S, Kneller J, Zou R, Leon LJ. Mechanisms of termination of atrial fibrillation by Class I antiarrhythmic drugs: evidence from clinical, experimental, and mathematical modeling studies. J Cardiovasc Electrophysiol. 2003;14:S133–S139. doi: 10.1046/j.1540.8167.90302.x. [DOI] [PubMed] [Google Scholar]
- 39.Sicouri S, Talarico M, Antzelevitch C. Antiarrhythmic effects of losartan and enalapril in canine pulmonary vein sleeves. Heart Rhythm. 2009;6:S91. doi: 10.1111/j.1540-8167.2010.01972.x. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]


