Only months from now, another decade will dawn on stroke research with a dearth of therapeutic innovations developed to reverse the devastating effects of acute ischemic stroke. Despite generations of academic research investigators and associated industry collaborations, our clinical trials have marked each decade by successive eras of failed treatments, with only a few exceptions. The recent publication of results from the Desmoteplase In Acute ischemic Stroke (DIAS)-2 trial provides important lessons that may hold the clues to reversing stroke in the 2010s.1 DIAS-2 was undoubtedly a landmark study, incorporating sophisticated trial design with the latest innovations in CT and MRI techniques to confirm benefit of a novel fibrinolytic agent to treat acute ischemic stroke up to 9 hours after symptom onset. The potential for another stroke therapy in clinical practice was replaced by collective introspection on the determinants of yet another negative trial following earlier positive results.2 Once again, an article commences by underscoring the limited treatment options for stroke, details the saga of an elaborate yet negative clinical trial, and ends by noting that the “criteria to optimise the selection and treatment outcomes” remain elusive. The perpetual motion of academia echoes the familiar chorus that further studies are indicated, but perhaps this philosophical juncture provides an opportunity to heed important lessons learned. An endless list of reasons can be cited for failure, as in the litany of articles on neuroprotective trials or the thoughtful considerations of STAIR,3, 4 yet most are difficult to prove or easily remedy without a comprehensive overhaul. Overall, DIAS-2 teaches us that the traditional approaches to stroke therapy may require revision of our currently engrained textbook approach to key variables such as time, imaging of the penumbra, ischemic pathophysiology, predictors of outcome, and the paradigm of randomized, controlled trials (RCTs) to prove efficacy of a single intervention. Tweaking such aspects may translate into therapeutic breakthroughs and allow us to reflect upon a very different decade of stroke research by 2020.
Time was a key focus of DIAS-2, employing an extended window from 3 to 9 hours after symptom onset to test a novel fibrinolytic drug for late reperfusion. As in the recent success of ECASS III with thrombolysis up to 4.5 hours after onset,5 offering treatment beyond the strict and perhaps arbitrary time limit of 3 hours has been a critical goal. “Time is brain” may be a useful slogan to accelerate triage of acute stroke, but such dogma defies the vascular pathophysiology of cerebral blood flow. Faster treatment always seems better, yet hemodynamics and the timecourse of collateral perfusion and subsequent failure vary substantially across individuals.6, 7 Time from symptom onset is not synonymous with stroke onset, as it only reflects when collaterals become insufficient to sustain normal neurological function. The timecourse of collateral failure and clinical deterioration vary and are influenced by numerous factors such as lesion location. In fact, patient presentation to the emergency room may be driven by such features as many are often dissuaded from seeking medical attention due to fluctuation or mild symptoms. Starting the clock in such cases remains a challenge. Reperfusion of mismatch at later timepoints, as in DIAS-2, may also not translate into substantial clinical improvement as these “survivors” may have tolerated prolonged hypoperfusion with only modest impairment. Interestingly, the DIAS-2 investigators found no difference in response rates based on time, an emerging pattern evidenced with other stroke interventions despite several prior studies to the contrary, raising questions about the accuracy of defining time windows without imaging.
Imaging may reveal why time is important, disclosing facets that relate to vascular changes such as no reflow and endothelial ischemia, aside from evolving ischemic injury in tissue. DIAS-2 highlighted multimodal CT and MRI, including parenchymal images with both angiography and perfusion measures. These techniques have rapidly gained traction in routine clinical practice, yet equating mismatch with penumbra may be especially misleading if CT and MRI approaches are incongruent. Real-time evaluation of perfusion on MRI typically employs time-domain parameters whereas cerebral blood volume is most often used on CT. These perfusion measures reflect distinct aspects of flow, much as CT and MRI angiography emphasize different aspects of vascular occlusion. Given such differences in technique, the marked differences in CT and MRI measures of mismatch and variability in core estimates noted in DIAS-2 are no surprise. If any given subject in DIAS-2 was imaged with both CT and MRI concurrently, mismatch volumes would be inconsistent, almost guaranteeing discordance across the entire population. No explanation was provided for the 43% core lesion growth in MRI cases, yet 44.7% diminution in the CT cases of the highest dose tier.1 DIAS-2 has convinced some that the use of imaging is superfluous and others have abandoned perfusion imaging in favor of vessel occlusion alone, assuming even marginal adjacent penumbra. Even TIMI 0–1 occlusive lesions were dissimilar on CT and MRI (p=0.04),1 likely reflecting the techniques as much as vascular pathology. The exact degree of mismatch may also be irrelevant unless one considers specific parameters such as CBV and the underlying vascular pathophysiology. CBV has a biphasic course, rising early with subsequent demise, and is often difficult to ascertain without proper delay correction. Although limited CT perfusion coverage was impugned, many of the imaging flaws may rest with interpretation and not acquisition. Multiparametric perfusion analyses will likely elucidate such complexities in future studies and differences in collateral perfusion beyond a progressive atherosclerotic lesion versus abrupt cardioembolic occlusion must be considered.8 The wealth of information provided by imaging allows rapid identification of a causative lesion and mechanism in a particular case yet observations across a cohort may be more elusive.
Ischemic pathophysiology may be redefined by imaging in trials such as DIAS-2. Even after conclusion of a negative trial, imaging provides an opportunity to realize subgroup or subtype distinctions. Summary statistics on clinical endpoints may not reflect important biological responses. Certain vascular lesions may respond preferentially to a drug like desmoteplase. Alternatively, this fibrinolytic drug may be futile with residual hypoperfusion and no proximal or even demonstrable thrombotic occlusion. Infusion of a lytic agent may actually be harmful if such hypoperfusion is due to downstream resistance in the microcirculation and venous system. Much needs to be learned about the heterogeneity and complexity of collateral perfusion. Even within the prolonged window of DIAS-2, these early phases of ischemia dominated by hemodynamics provide clues to reversing ischemic injury. The relatively low median NIHSS score of 9 with a middle cerebral artery occlusion suggests that robust collateral perfusion may be sufficient to ward off neurological dysfunction. Furthermore, the lack of increased bleeding with desmoteplase underscores that hemorrhagic transformation results from severe ischemia and not just exposure to thrombolytics. The effect of a particular drug or device on ischemia may be discerned beyond simply noting that the investigational treatment altered baseline prognostic factors.
In recent years, we have learned that baseline prognostic factors such as age, gender, NIHSS score or serum glucose may be extremely influential but few studies have answered why. In DIAS-2, careful selection and randomization attempted to control for such influences and it was still noted that women do worse. Restrictive selection criteria in trials may exclude many to find an ideal population far removed from the workaday practice of stroke yet generalizability is paramount. Larger studies are often demanded to overcome “inadequate sample sizes” to detect any benefit of a drug or device beyond baseline prognostic variables. Few investigators have pursued the pathophysiology of why these variables often seem insurmountable yet such knowledge, as in the detrimental consequences of hyperglycemia,9 may yield further avenues for treatment. Predicting outcomes has likely been oversimplified as sequential models may be needed to relate baseline features including imaging with early changes in vascular status, resultant tissue fate, and ultimately clinical outcome at day 90. DIAS-2 once again reiterates the fact that medical management, prevention of complications such as early recurrent stroke, use of rehabilitation, and withdrawal of care may dramatically alter outcomes far beyond the trialed intervention.
Consideration of these myriad variables that affect the clinical course of a stroke patient from onset to day 90 poses the provocative question of what it is we are testing in a clinical trial. One may assume that DIAS-2 is about desmoteplase alone, yet perhaps meticulous attention to and controlling for other variables sways overall trial results. When both cohorts do well, one may conclude that DIAS-2 is a positive trial, championing state-of-the-art medical management of acute stroke. Isolated drug or device interventions alone may have marginal impact, challenging the current paradigm of most RCTs. Replication of trial results in clinical practice is contingent on comprehensive stroke care and this should be monitored in registries. Given the heterogeneity of acute ischemic stroke, a promising drug such as desmoteplase may work differently in a proximal versus distal occlusion. The milder strokes, smaller cores, less mismatch, and markedly fewer proximal occlusions may explain the failure of DIAS-2 after DEDAS.2 Drug and device cocktails short of the kitchen sink approach may also be necessary in a particular case, suggesting that our trials should be honed around diagnosis rather than a particular therapy. Tailored stroke therapy or N-of-1 trials may yield greater success than one drug/device fits all.10
DIAS-2 highlighted advances in imaging and medical management of acute stroke that may enhance clinical trials and underscored fundamental issues that tantalize. Instead of asking what went wrong we should realize that reversing stroke in the 2010s is far from a pipe dream and perhaps only a few, yet large steps away.
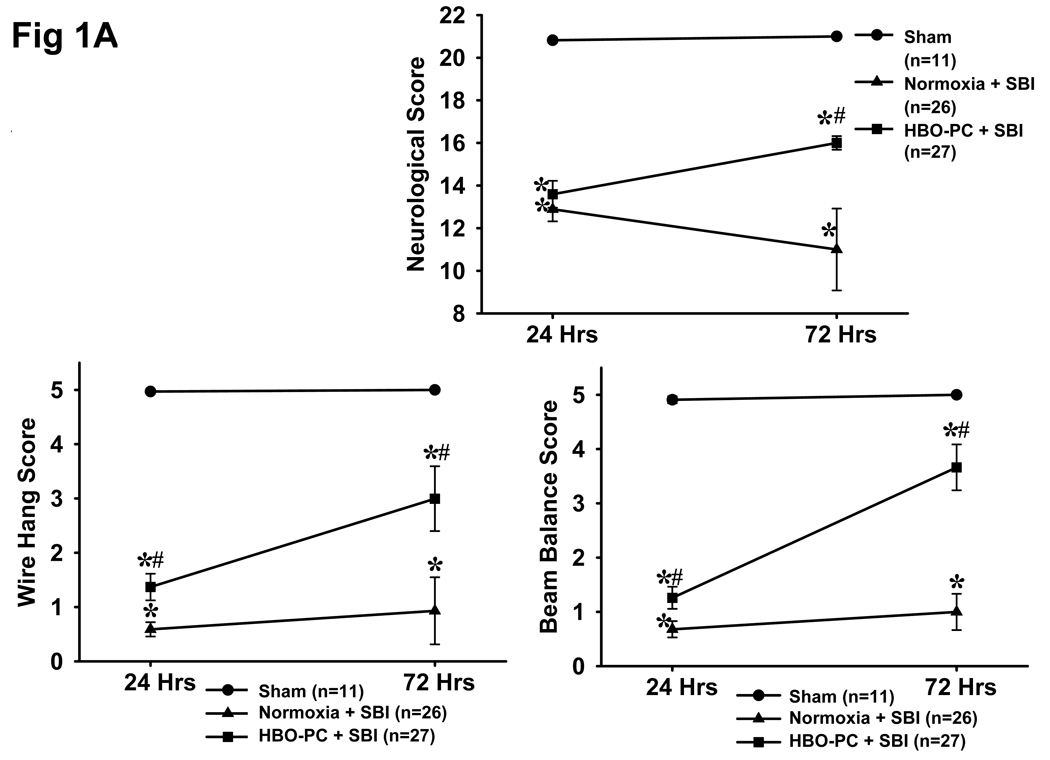
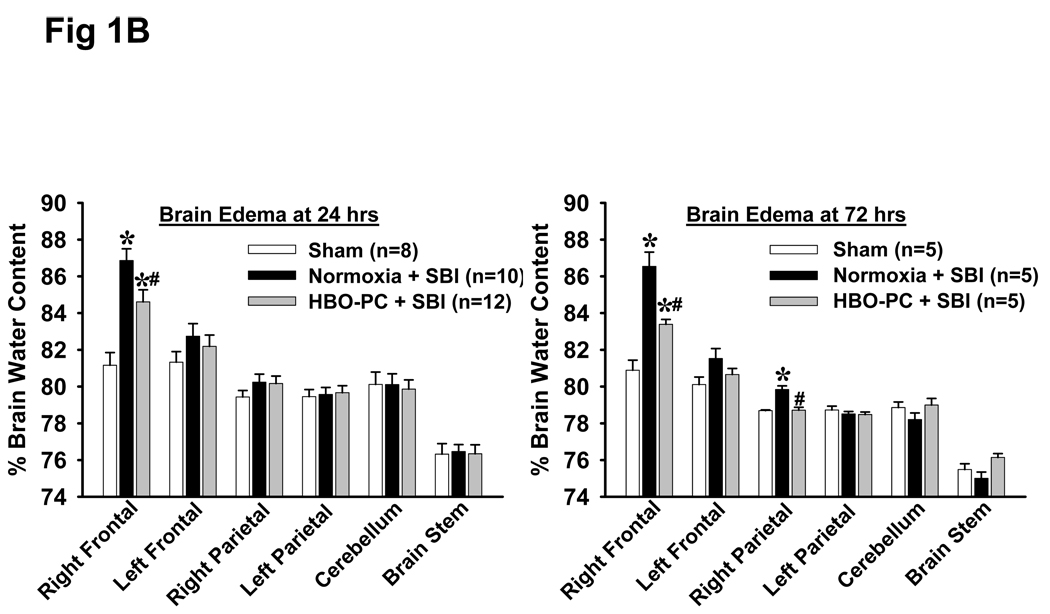
Fig 1.
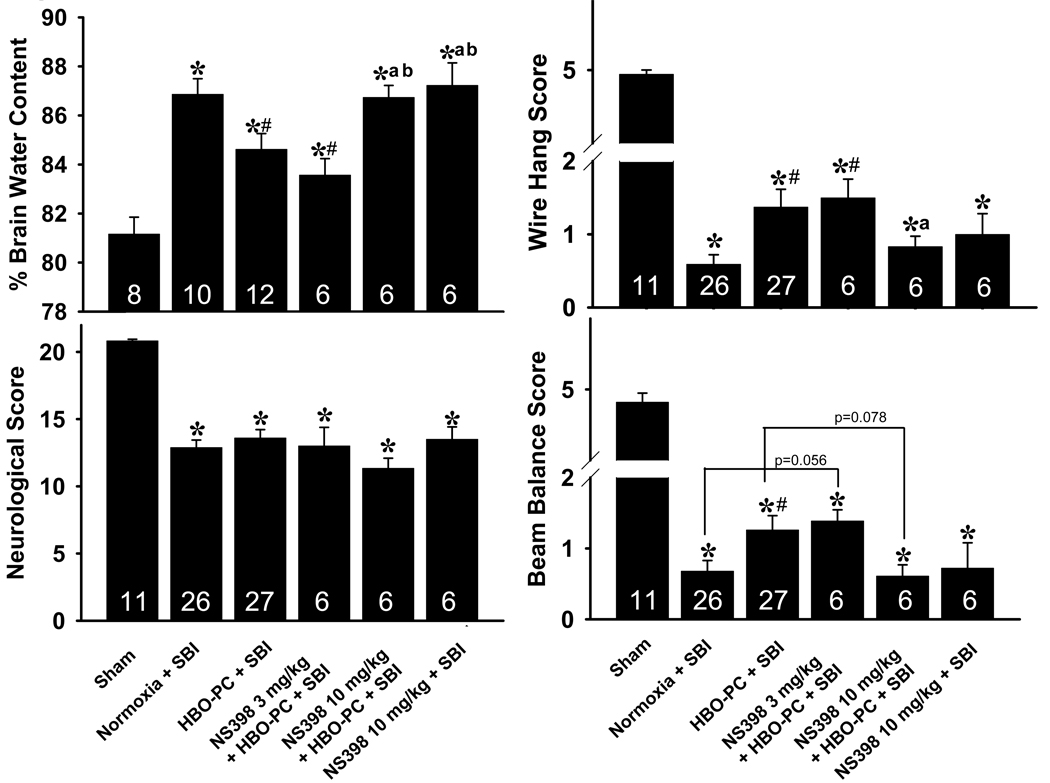
A: HBO Preconditioning Ameliorates Neurological Deficits
B: HBO Preconditioning Reduces Brain Edema
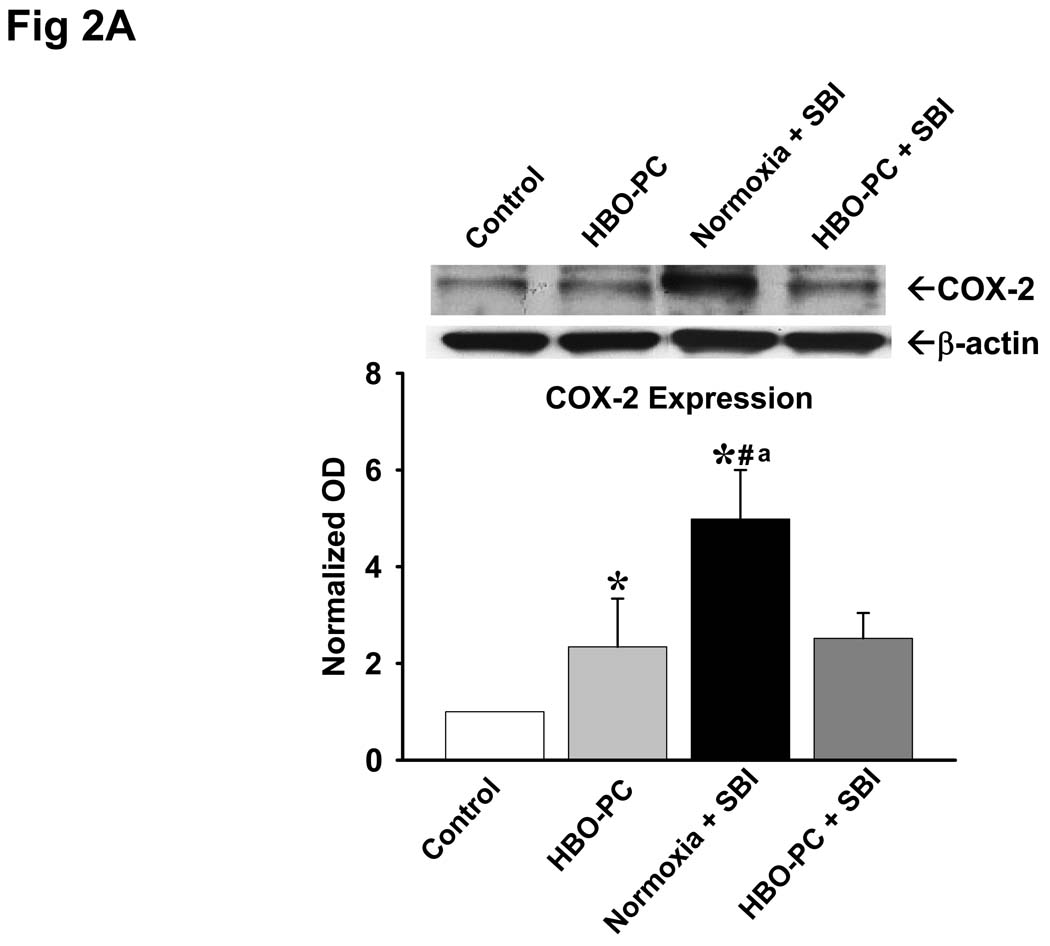
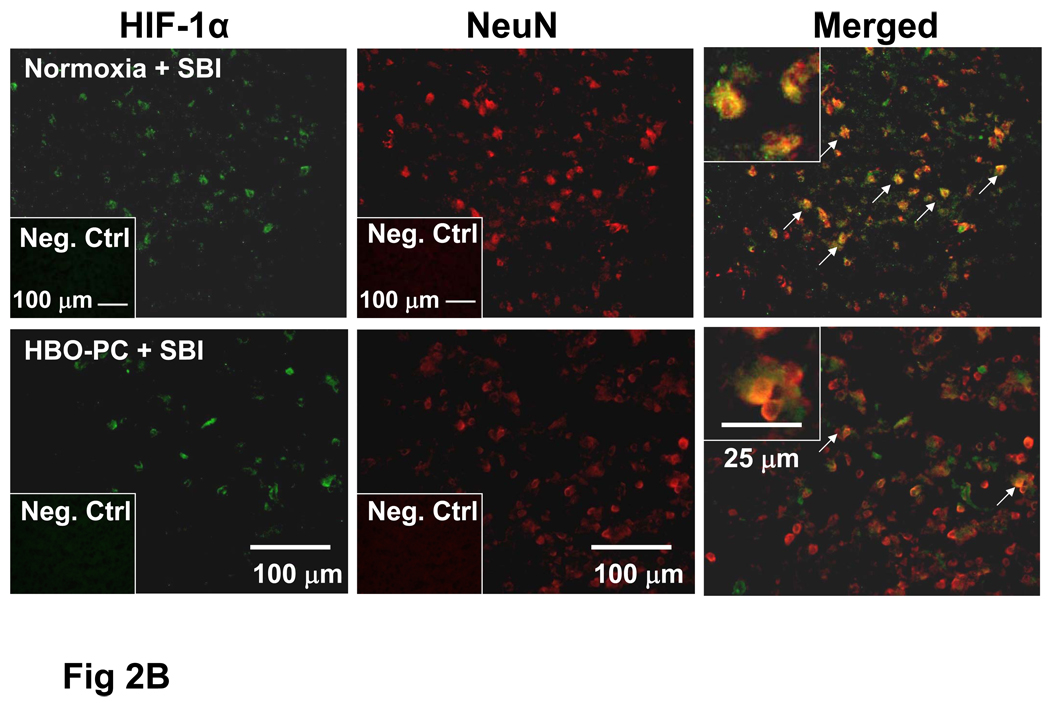
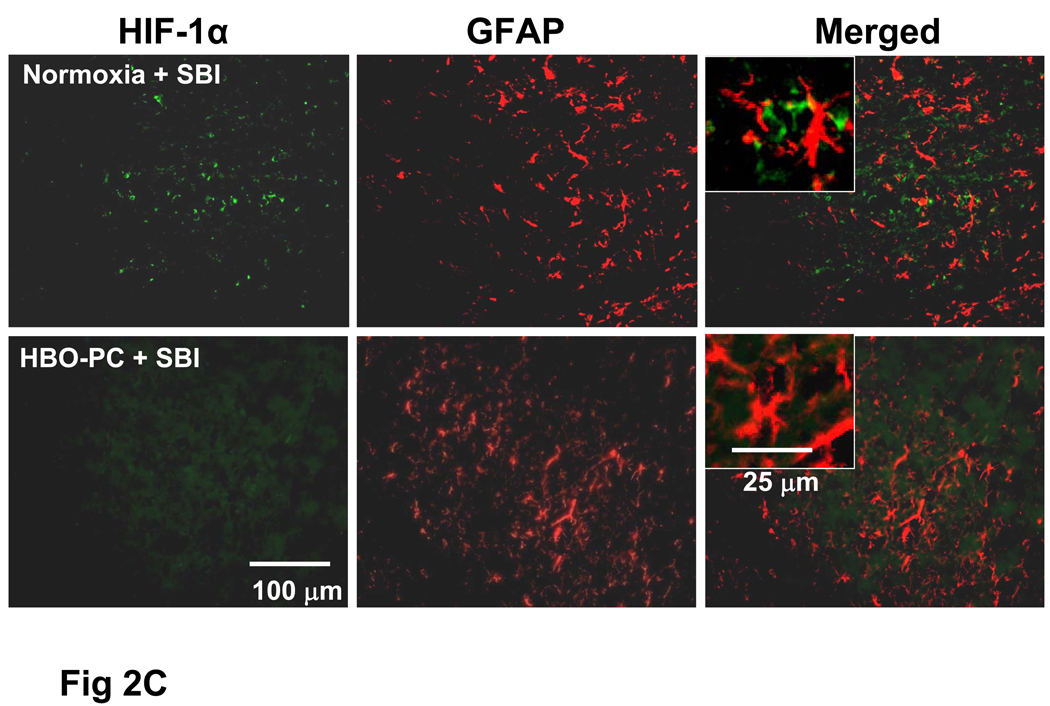
Fig 2.
A: Effects of SBI and HBO Preconditioning on Cyclooxygenase-2 Expression
B: SBI Induced HIF-1α Upregulation in Neurons is Attenuated by HBO-PC
C: HIF-1α is Not Co-localized with Astrocytic Marker
Fig 3.
Suboptimal Dose of COX-2 Inhibitor Reverses HBO-PC Induced Neuroprotection
References
- 1.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, Kaste M, Lipka LJ, Pedraza S, Ringleb PA, Rowley HA, Schneider D, Schwamm LH, Leal JS, Sohngen M, Teal PA, Wilhelm-Ogunbiyi K, Wintermark M, Warach S. Intravenous desmoteplase in patients with acute ischaemic stroke selected by mri perfusion-diffusion weighted imaging or perfusion ct (DIAS-2): A prospective, randomised, double-blind, placebocontrolled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, Sachara C, Soehngen M, Warach S, Hacke W. Dose escalation of desmoteplase for acute ischemic stroke (dedas): Evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009 doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher M, Hanley DF, Howard G, Jauch EC, Warach S. Recommendations from the stair v meeting on acute stroke trials, technology and outcomes. Stroke. 2007;38:245–248. doi: 10.1161/01.STR.0000255951.37434.aa. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–265. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]
- 7.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH, Jeon P, Chung CS, Lee KH, Alger JR, Liebeskind DS. Mr mismatch profiles in patients with intracranial atherosclerotic stroke: A comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M. Impact of admission hyperglycemia on stroke outcome after thrombolysis: Risk stratification in relation to time to reperfusion. Stroke. 2004;35:2493–2498. doi: 10.1161/01.STR.0000143728.45516.c6. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Keller JL, Jaeschke R, Rosenbloom D, Adachi JD, Newhouse MT. The n-of-1 randomized controlled trial: Clinical usefulness. Our three-year experience. Ann Intern Med. 1990;112:293–299. doi: 10.7326/0003-4819-112-4-293. [DOI] [PubMed] [Google Scholar]