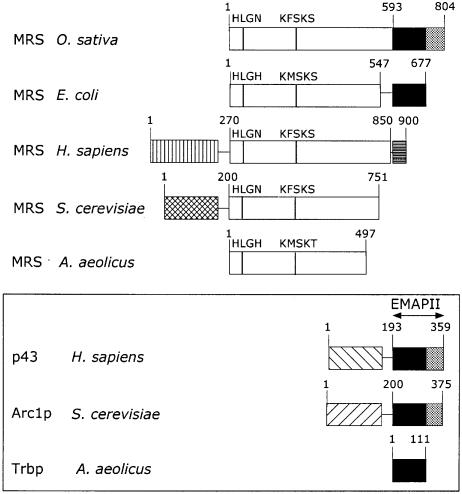
Fig. 1. The five groups of MetRS and alignment of the EMAPII related RNA binding domains. Only one member of each group is indicated. A more detailed list of MetRSs related to these groups is included in the main text. The open boxes indicate the conserved minimal domain of MetRS, as found in the eubacterium A.aeolicus. MetRS from O.sativa displays an additional C-terminal domain related to the EMAPII moiety of Homo sapiens p43, also recovered in S.cerevisiae Arc1p. The first part of this domain, indicated as a black box, is appended to E.coli MetRS. It corresponds to a dimeric tRNA binding protein (Trbp111) recovered in A.aeolicus. MetRSs from H.sapiens and S.cerevisiae possess N-terminal polypeptide extensions involved in their association with the multisynthetase complex or Arc1p, respectively. The human enzyme has an additional C-terminal polypeptide unrelated to EMAPII or Trbp111 motifs, corresponding to a RNA binding motif found as repeated units in the multifunctional GluProRS. The two conserved functional motifs related to HIGH and KMSKS sequences of MetRSs are indicated. Numbers refer to amino acid residues.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.